
- Chemistry - Home
- Chemistry - Introduction
- Chemistry - Branches
- Chemistry - Radioactivity
- Chemistry - Nuclear Energy
- Chemistry - Metals
- Chemistry - Metallurgy
- Chemistry - Sodium
- Chemistry - Calcium
- Chemistry - Aluminum
- Chemistry - Magnesium
- Chemistry - Maganese
- Chemistry - Iron
- Chemistry - Copper
- Chemistry - Silver
- Chemistry - Gold
- Chemistry - Platinum
- Chemistry - Zinc
- Chemistry - Mercury
- Chemistry - Plutonium
- Chemistry - Uranium
- Chemistry - Lead
- Chemistry - Thorium
- Chemistry - Hydrogen
- Chemistry - Helium
- Chemistry - Oxygen
- Chemistry - Carbon
- Chemistry - Nitrogen
- Chemistry - Chemical Law
- Chemistry - Discovery of Elements
- Elements With Their Valence
- Elements With Their Atomic Number
- Chemistry - Nobel Prize
- Chemistry Part 2 - Online Quiz
- Chemistry Part 2 - Online Test
- Chemistry Part 2 - Quick Guide
- Chemistry - Useful Resources
- Chemistry - Discussion
Chemistry - Oxygen
Introduction
Oxygen is the member of group 16 on the periodic table; however, most of the time, it is treated differently from its group.
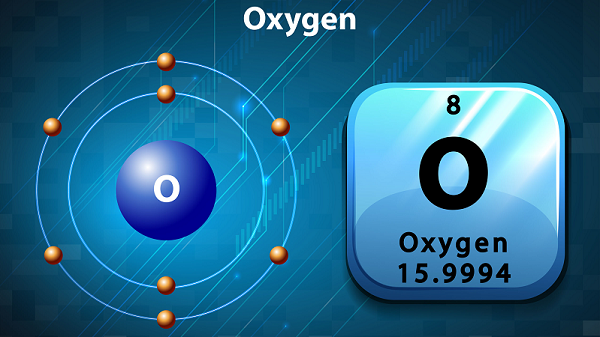
The symbol of oxygen is O and atomic number is 8.
Oxygen has about nine allotropes and the most common allotrope is diatomic oxygen (i.e. O2). Other important allotrope is Ozone i.e. O3.
Oxygen, first time, was noticed by Swedish pharmacist Carl Wilhelm Scheele.
Salient Features of Oxygen
Oxygen is characteristically categorized as the member of chalcogen group.
The word "chalcogen" is derived from a Greek word khalks, which means copper and the Latin-Greek word Gens, which means born or produced.
Oxygen is a highly reactive gas (or nonmetallic element); hence, it is an oxidizing agent that readily forms oxides with most of the elements and compounds.
Oxygen has six valence electrons.
The melting point of oxygen is -218.80C and the boiling point is -1830C.
Occurrence of Oxygen
With about 20.8 percent share (in total earths atmospheric constituents), oxygen is the second ranked element of the earths atmosphere.
Oxygen occurs almost in sphere of the earth namely atmosphere, hydrosphere, and lithosphere.
During the photosynthesis process, free oxygen is produced by all green plants.
Oxygen occurs as constituent copper ores.
A human body contains about 65 percent oxygen.
By mass, almost half of the earths crust is composed of oxygen (i.e. its oxides).
By mass, oxygen is the third-most abundant element that found in the universe; the first and second are hydrogen and helium accordingly.
Oxygen (i.e. O2) is a colorless and odorless diatomic gas.
Oxygen dissolves in water very easily; however, the solubility of oxygen in the water is temperature-dependent.
Compounds of Oxygen
Following are the major compounds of oxygen −
Oxide
Peroxide
Carbon dioxide - CO2
Hydroxide - OH-
Ozone - O3
Mercury (II) oxide - HgO
Chlorate - ClO3
Aluminum oxide - Al2O3
Carbon monoxide - CO
Hypochlorite - ClO-
Silicon dioxide - SiO2
Hypofluorous acid - HOF
Sodium peroxide - Na2O2
Potassium chlorate - KClO3
Oxygen difluoride - OF2
Sodium oxide - Na2O
Uses of Oxygen
Oxygen (O2) is the most essential requirements for the respiration, without it, life cannot be imagined.
Oxygen is used in medicine.
Oxygen therapy is typically used to treat some diseases, such as, emphysema, pneumonia, some heart disorders, etc.
Some of the underwater activities, such as scuba diving, submarines, etc. also use artificial oxygen.

Aircrafts, mountaineers, etc. also use artificial oxygen.
Oxygen is also used in some of the industries, e.g. smelting of iron ore into steel in this process, about 55% of oxygen is used.