
- Chemistry - Home
- Chemistry - Introduction
- Chemistry - Branches
- Chemistry - Radioactivity
- Chemistry - Nuclear Energy
- Chemistry - Metals
- Chemistry - Metallurgy
- Chemistry - Sodium
- Chemistry - Calcium
- Chemistry - Aluminum
- Chemistry - Magnesium
- Chemistry - Maganese
- Chemistry - Iron
- Chemistry - Copper
- Chemistry - Silver
- Chemistry - Gold
- Chemistry - Platinum
- Chemistry - Zinc
- Chemistry - Mercury
- Chemistry - Plutonium
- Chemistry - Uranium
- Chemistry - Lead
- Chemistry - Thorium
- Chemistry - Hydrogen
- Chemistry - Helium
- Chemistry - Oxygen
- Chemistry - Carbon
- Chemistry - Nitrogen
- Chemistry - Chemical Law
- Chemistry - Discovery of Elements
- Elements With Their Valence
- Elements With Their Atomic Number
- Chemistry - Nobel Prize
- Chemistry Part 2 - Online Quiz
- Chemistry Part 2 - Online Test
- Chemistry Part 2 - Quick Guide
- Chemistry - Useful Resources
- Chemistry - Discussion
Chemistry - Magnesium
Introduction
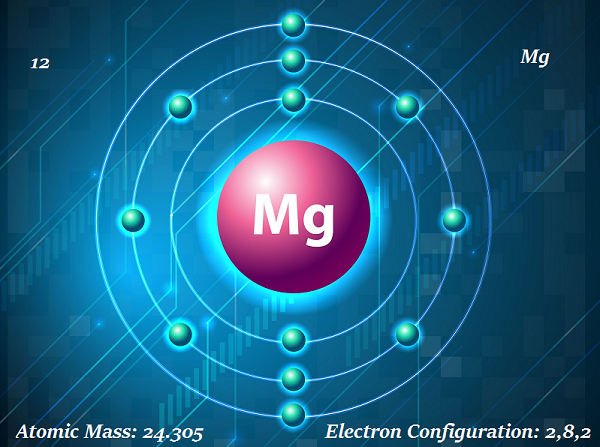
Magnesium is a shiny gray solid element.
The symbol of magnesium is Mg and atomic number is 12.
With approximately 80% of the world market share, China is the largest supplier of magnesium.
Salient Features of Magnesium
The density of magnesium is two-thirds the density of aluminum.
Among all the alkali metals of the Earth, magnesium has lowest melting point (i.e. about 1,2020F) and lowest boiling point (about 1,9940F).
Magnesium usually reacts with water at room temperature.
Sometimes, magnesium is also used as an igniter for thermite.
Magnesium, when burns in air, produces a brilliant-white light, which also includes strong ultraviolet wavelengths.

Magnesium, when burns, it produces intense bright and white light (see image given above).
Occurrence of Magnesium
By mass, magnesium is the eighth-most-abundant element found in the Earth's crust.
Magnesium is found usually in large deposits of magnesite, dolomite, and other such minerals.
The soluble magnesium ion is found in the mineral water.
After sodium and chlorine, magnesium is the third most abundant element dissolved in seawater.
Magnesium naturally occurs only in combination with some other elements.
By mass, magnesium is the 11th most abundant element in the human body and it is essential to all cells and enzymes.
Magnesium ions frequently interact with polyphosphate compounds including ATP, DNA, and RNA.
Compounds of Magnesium
Following are the major compounds of magnesium −
Magnesium carbonate - MgCO3
Magnesium chloride - MgCl2
Magnesium citrate - C6H6MgO7
Magnesium hydroxide - Mg(OH)2
Magnesium oxide - MgO
Magnesium sulfate - MgSO4
Magnesium sulfate heptahydrate - (MgSO47H2O)
Magnesium sulfate heptahydrate is commonly known as Epsom salt.
Usages of Magnesium
Magnesium has wide range of usage in our lives; however, some significant usages of magnesium are −
After iron and aluminum, magnesium is third most commonly used element.
Magnesium is especially used in super-strong, lightweight materials, and alloys.
Magnesium is also used as engine materials in the aircraft industry.
Magnesium is also used to purify the solvents; such as in preparing the super-dry ethanol.
Many of the automotive big brands including Mercedes, Porsche, BMW, Volkswagen, Chevrolet, etc. use magnesium in making their highly quality cars.
Because of having low weight and good electrical and mechanical properties, magnesium is commonly used in manufacturing laptops and tablet computers, mobile phones, cameras, and many other electronic components.
Magnesium sulfite is usually used in manufacturing paper.