
- Chemistry - Home
- Chemistry - Introduction
- Chemistry - Branches
- Chemistry - Radioactivity
- Chemistry - Nuclear Energy
- Chemistry - Metals
- Chemistry - Metallurgy
- Chemistry - Sodium
- Chemistry - Calcium
- Chemistry - Aluminum
- Chemistry - Magnesium
- Chemistry - Maganese
- Chemistry - Iron
- Chemistry - Copper
- Chemistry - Silver
- Chemistry - Gold
- Chemistry - Platinum
- Chemistry - Zinc
- Chemistry - Mercury
- Chemistry - Plutonium
- Chemistry - Uranium
- Chemistry - Lead
- Chemistry - Thorium
- Chemistry - Hydrogen
- Chemistry - Helium
- Chemistry - Oxygen
- Chemistry - Carbon
- Chemistry - Nitrogen
- Chemistry - Chemical Law
- Chemistry - Discovery of Elements
- Elements With Their Valence
- Elements With Their Atomic Number
- Chemistry - Nobel Prize
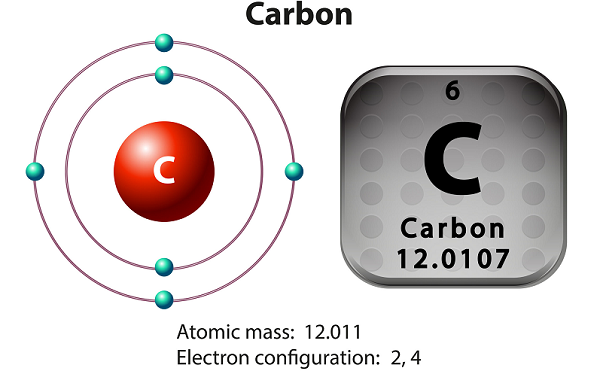
Chemistry - Carbon
Introduction
Carbon is a non-metallic and tetravalent element.
Tetravalent means carbon makes four electrons available to form the covalent chemical bonds.
Carbon has three isotopes that occur naturally namely 12C, 13C, and 14C.
Among them, 12C and 13C are stable, but 14C is a radioactive isotope. Half-life of 14C is about 5,730 years.
Salient Features of Carbon
The physical properties of carbon largely depend on its allotropes.
Major allotropes of carbon are graphite, diamond, and amorphous carbon.
Graphite is opaque, black, and very soft; hence, it used to form a streak on the paper.
Diamond very hard (the hardest naturally occurring material) and transparent.
Graphite is a good conductor of electricity.
Diamond is bad conductor of electricity.
Carbon most likely has the highest sublimation point among all the elements.
Occurrence of Carbon
In terms of mass, carbon is the fourth most abundant chemical element found in the universe (after hydrogen, helium, and oxygen).
Carbon is available in abundance in the Sun, stars, comets, and in the atmospheres of most of the planets.
Carbon is found in the earths atmosphere and dissolved in water.
Hydrocarbons, such as coal, petroleum, and natural gas, all of them contain carbon.
Carbon is also found in methane hydrates, which found in polar regions and under the seas.
Some of the rocks enriched of carbon are coal, limestone, dolomite, etc.
Coal is very rich in carbon; hence, it is the largest commercial source of mineral carbon.
Coal shares about 4,000 gigatonnes or 80% of total fossil fuel.
Compounds of Carbon
Following are the major compounds of Carbon −
Cyanogen - CN2
Hydrogen cyanide - HCN
Cyanamide - CN2H2
Isocyanic acid - HNCO
Cyanogen chloride - CNCl
Chlorosulfonyl isocyanate - CNClO3S
Cyanuric chloride - NCCl3
Carbon disulfide - CS2
Carbonyl sulfide - OCS
Carbon monosulfide - CS
Uses of Carbon
Depending upon the allotrops, carbon is used in range of applications.
Carbon is one of the most essential elements of life without it, we cannot imagine life on the earth.
The fossil fuel namely methane gas and crude oil (petroleum), coal etc. are used in everyday life.
Graphite, combining with clay, used in making 'lead' used in pencils.
Charcoal is also used as a drawing material in artwork, iron smelting, barbecue grilling, etc.
Diamond is usually used in jewelry.
Industrial diamonds are used in cutting, drilling, and polishing tools for machining the metals and stone.
Fossil hydrocarbons, and carbon fiber are used in making plastic.