
 Data Structure
Data Structure Networking
Networking RDBMS
RDBMS Operating System
Operating System Java
Java MS Excel
MS Excel iOS
iOS HTML
HTML CSS
CSS Android
Android Python
Python C Programming
C Programming C++
C++ C#
C# MongoDB
MongoDB MySQL
MySQL Javascript
Javascript PHP
PHP
- Selected Reading
- UPSC IAS Exams Notes
- Developer's Best Practices
- Questions and Answers
- Effective Resume Writing
- HR Interview Questions
- Computer Glossary
- Who is Who
Modelling the Otto and Diesel Cycles in Python
Otto Cycle
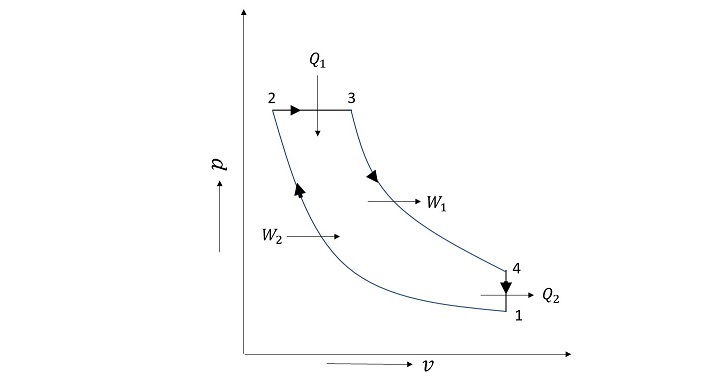
An air standard cycle called the Otto Cycle is employed in spark ignition (SI) engines. It comprises of two reversible adiabatic processes and two isochoric processes (constant volume), totaling four processes. When the work interactions take place in reversible adiabatic processes, the heat addition (2-3) and rejection (4-1) occur isochorically (3-4 and 1-2). The Otto cycle's schematic is shown in Figure given below.

To model the cycle in Python, the input variables considered are maximum pressure $\mathrm{(P_{max})}$, minimum pressure $\mathrm{(P_{min})}$, maximum volume $\mathrm{(V_{max})}$, compression ratio (r), and adiabatic exponent $\mathrm{(\gamma)}$. Table 2 explains the thermodynamic computations of different processes involved in the Otto cycle
Process 1-2
$$\mathrm{p_{1} \: = \: p_{min}}$$
$$\mathrm{v_{1} \: = \: v_{max}}$$
Using compression ratio (?), first the volume at point 2 will be evaluated based on the volume at point 1 as
$$\mathrm{v_{2} \: = \: \frac{v_{1}}{r}}$$
Then the adiabatic constant along the process 1-2 is evaluated as
$$\mathrm{c_{1} \: = \: p_{1} \: \times \: v_{1}^{\gamma}}$$
Once $\mathrm{c_{1}}$ is known, the pressure variation along the line 1-2 is evaluated as
$$\mathrm{p \: = \: \frac{c_{1}}{v^{\gamma}}}$$
Process 2-3
$$\mathrm{p_{3} \: = \: p_{max}}$$
As the process is isochoric, so the volume remains the same thus
$$\mathrm{v_{3} \: = \: v_{2}}$$
Therefore, the pressure at point 2 can be evaluated as
$$\mathrm{p_{2} \: = \: \frac{c_{1}}{v^{\gamma}_{2}}}$$
Process 3-4
Let $\mathrm{c_{2}}$ be the constant along line 3-4. As pressure and temperature at point 3 are known, so the constant along the reversible adiabatic line can be evaluated as
$$\mathrm{c_{2} \: = \: p_{3} \: \times \: v_{3}^{\gamma}}$$
And as $\mathrm{v_{4} \: = \: v_{1}}$, so the pressure along 3-4 can be evaluated as
$$\mathrm{p \: = \: \frac{c_{2}}{v^{\gamma}}}$$
Process 4-1
$\mathrm{c_{2}}$ and $\mathrm{c_{4}}$ are already known so $\mathrm{p_{4}}$ can be evaluated as
$$\mathrm{p_{4} \: = \: \frac{c_{4}}{v^{\gamma}_{4}}}$$
Python Program for the Otto Cycle
The Python function for the Otto cycle is as follows
from pylab import *
from pandas import *
def otto(p_min,p_max,v_max,r,gma):
font = {'family' : 'Times New Roman','size' : 39}
figure(figsize=(20,15))
rc('font', **font)
'''This function prints Otto cycle
arguments are as follows:
_min: minimum pressure
p_max: Maximum pressure
v_max: Maximum volume
r: compression ratio
gma: Adiabatic exponent
The order of arguments is:
p_min,p_max,v_max,r,gma
'''
#~~~~~~~~~~~~~~~~~~~~~~~~~
# Process 1-2
#~~~~~~~~~~~~~~~~~~~~~~~~~
p1=p_min
v1=v_max
v2=v1/r
c1=p1*v1**gma
v=linspace(v2,v1,100)
p=c1/v**gma
plot(v,p/1000,'b',linewidth=3)
#~~~~~~~~~~~~~~~~~~~~~~~~~
# Process 2-3
#~~~~~~~~~~~~~~~~~~~~~~~~~
p3=p_max
v3=v2
p2=c1/v2**gma
p=linspace(p2,p3,100)
v=100*[v3]
plot(v,p/1000,'r',linewidth=3)
#~~~~~~~~~~~~~~~~~~~~~~~~~
# Process 3-4
#~~~~~~~~~~~~~~~~~~~~~~~~~
c2=p3*v3**gma
v4=v1
v=linspace(v3,v4,100)
p=c2/v**gma
plot(v,p/1000,'g',linewidth=3)
#~~~~~~~~~~~~~~~~~~~~~~~~~
# Process 4-1
#~~~~~~~~~~~~~~~~~~~~~~~~~
v4=v1
p4=c2/v4**gma
p=linspace(p1,p4,100)
v=100*[v1]
plot(v,p/1000,'r',linewidth=3)
title('Otto Cycle',size='xx-large',color='k')
xlabel('Volume ($m^3$)')
ylabel('Pressure (kPa)')
text(v1,p1/1000-30,'1')
text(v2,p2/1000-200,'2')
text(v3+0.01,p3/1000-20,'3')
text(v4,p4/1000+10,'4')
data={'p':[p1,p2,p3,p4],
'v':[v1,v2,v3,v4],
'c':[c1,'' ,c2,'' ],
'State': [1,2,3,4]}
df=DataFrame(data)
savefig('Otto_final.jpg')
return df.set_index('State')
oc=otto(2*10**5,35*10**5,0.5,5,1.4)
show()
oc
For $\mathrm{p_{min} \: = \: 2 \: \times \: 10^{5} \: Pa \: , \: p_{max} \: = \: 35 \: \times \: 10^{5} \: Pa \: , \: v_{max} \: = \: 0.5 \: m^{3} \: , \: r \: = \: 5 \: and \: \gamma \: = \: 1.4 \: ,}$ the program generated Otto cycle plot is as follows
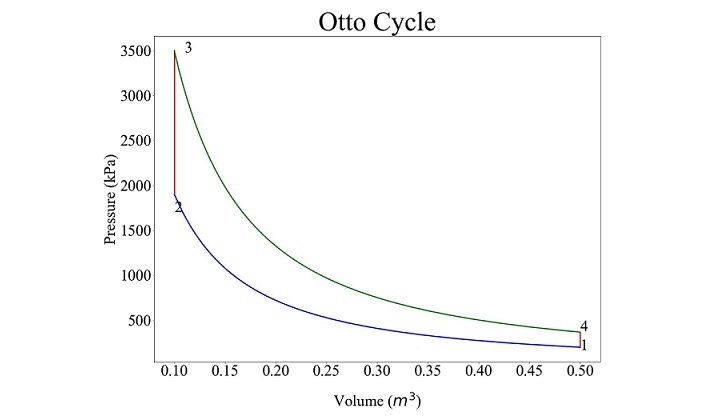
The pressures and volumes at different points obtained from the code is as follows
State |
p |
v |
|---|---|---|
1 |
2.000000e+05 |
0.5 |
2 |
1.903654e+06 |
0.1 |
3 |
3.500000e+06 |
0.1 |
4 |
3.677139e+05 |
0.5 |
Diesel Cycle
An air standard cycle used in compression ignition (CI) engines is the diesel cycle. Four processes make up the cycle two reversible adiabatic, one isobaric (constant pressure), and two isochoric (constant volume). Heat addition happens in process 2-3, whereas heat rejection happens in process 4-1. The processes 1-2 and 3-4, respectively, are where the work interaction to and from the cycle takes place. The Diesel cycle diagram is shown in Figure given below.
To model the cycle, the input variables considered are maximum pressure $\mathrm{(p_{max})}$, minimum pressure $\mathrm{(p_{min})}$, maximum volume $\mathrm{(v_{max})}$, cut-off ratio $\mathrm{(r_{c})}$, and adiabatic exponent \mathrm{(\gamma)}. The thermodynamic computations of different processes involved in the Diesel cycle are explained below
Process 1-2
$$\mathrm{p_{1} \: = \: p_{min}}$$
$$\mathrm{v_{1} \: = \: v_{max}}$$
$$\mathrm{p_{2} \: = \: p_{max}}$$
Since 1-2 is an adiabatic process, so it follows $\mathrm{pv^{\gamma} \: = \: const \: ;}$ let the constant be $\mathrm{(c_{1})}$. The volume at point 2 can be evaluated as
$$\mathrm{v_{2} \: = \: v_{1} \: \times (\frac{p_{1}}{p_{2}})^{\frac{1}{\gamma}}}$$
Therefore $\mathrm{c_{1} \: = \: p_{1} \: \times \: v_{1}^{\gamma}}$
Then the adiabatic constant along the process 1-2 is evaluated as
$$\mathrm{c_{1} \: = \: p_{1} \: \times \: v_{1}^{\gamma}}$$
Once $\mathrm{c_{1}}$ is known, the pressure variation along the line 1-2 is evaluated as
$$\mathrm{p \: = \: \frac{c_{1}}{v^{\gamma}}}$$
Process 2-3
As the process is isobaric, so the pressure remains the same, thus
$$\mathrm{p_{3} \: = \: p_{2}}$$
The volume at point 3 can be evaluated as
$$\mathrm{v_{3} \: = \: r_{c} \: \times \: v_{2}}$$
So, the pressure variation between volumes $\mathrm{v_{2}}$ and $\mathrm{v_{3}}$ can be known easily.
Process 3-4
Let $\mathrm{c_{2}}$ be the constant along line 3-4. As pressure and temperature at point 3 are known, so the constant along the reversible adiabatic line can be evaluated as
$$\mathrm{c_{2} \: = \: p_{3} \: \times \: v_{3}^{\gamma}}$$
And as $\mathrm{v_{4} \: = \: v_{1}}$, so the pressure variation along 3-4 can be evaluated as
$$\mathrm{p \: = \: \frac{c_{2}}{v^{\gamma}}}$$
Process 4-1
$\mathrm{c_{2}}$ and $\mathrm{v_{4}}$ are already known, so $\mathrm{p_{4}}$ can be evaluated as
$$\mathrm{p_{4} \: = \: \frac{c_{4}}{v^{\gamma}_{4}}}$$
Python Program to Model the Diesel Cycle
The python function to model the Diesel cycle is as follows
#~~~~~~~~~~~~~~~~~~~
# Diesel Cycle
#~~~~~~~~~~~~~~~~~~~
def diesel(p_min,p_max,v_max,r_c,gma):
font = {'family' : 'Times New Roman','size' : 39}
figure(figsize=(20,15))
title('Rankine Cycle with Feed water heating (T-s Diagram)',color='b')
rc('font', **font)
'''This function prints Diesel cycle
arguments are as follows:
p_min: minimum pressure
p_max: Maximum pressure
v_max: Maximum volume
rc: Cut-Off ratio
gma: Adiabatic exponent
The order of arguments is:
p_min,p_max,v_max,rc,gma
'''
#~~~~~~~~~~~~~~~~~~~~~~~~~
# Process 1-2
#~~~~~~~~~~~~~~~~~~~~~~~~~
p1=p_min
v1=v_max
p2=p_max
v2=v1*(p1/p2)**(1/gma)
c1=p1*v1**gma
v=linspace(v2,v1,100)
p=c1/v**gma
plot(v,p/1000,'b',linewidth=3)
#~~~~~~~~~~~~~~~~~~~~~~~~~
# Process 2-3
#~~~~~~~~~~~~~~~~~~~~~~~~~
p3=p2
p=zeros(100)
p=p+p2
v3=r_c*v2
v=linspace(v2,v3,100)
plot(v,p/1000.,'r',linewidth=3)
#~~~~~~~~~~~~~~~~~~~~~~~~~
# Process 3-4
#~~~~~~~~~~~~~~~~~~~~~~~~~
v4=v1
c2=p3*v3**gma
v=linspace(v3,v4,100)
p=c2/v**gma
plot(v,p/1000,'g',linewidth=3)
#~~~~~~~~~~~~~~~~~~~~~~~~~
# Process 4-1
#~~~~~~~~~~~~~~~~~~~~~~~~~
v4=v1
v=100*[v4]
p4=c2/v4**gma
p=linspace(p1,p4,100)
plot(v,p/1000.,'m',linewidth=3)
title('Diesel Cycle',size='xx-large',color='b')
xlabel('Volume ($m^3$)')
ylabel('Pressure (kPa)')
text(v1,p1/1000-30,'1')
text(v2-0.01,p2/1000,'2')
text(v3+0.01,p3/1000-20,'3')
text(v4,p4/1000+10,'4')
data={'p':[p1,p2,p3,p4],
'v':[v1,v2,v3,v4],
'c':[c1,'' ,c2,'' ],
'State': [1,2,3,4]}
df=DataFrame(data)
savefig('Diesel_final.jpg')
return df.set_index('State')
dc=diesel(2*10**5,20*10**5,0.5,2,1.4)
show()
dc
For $\mathrm{p_{min} \: = \: 2 \: \times \: 10^{5} \: Pa \: , \: p_{max} \: = \: 20 \: \times \: 10^{5} \: Pa \: , \: v_{max} \: = \: 0.5 \: m^{3} \: , \: r_{c} \: = \: 2 \: and \: \gamma \: = \: 1.4 \: ,}$ the results obtained are shown in Figures given below
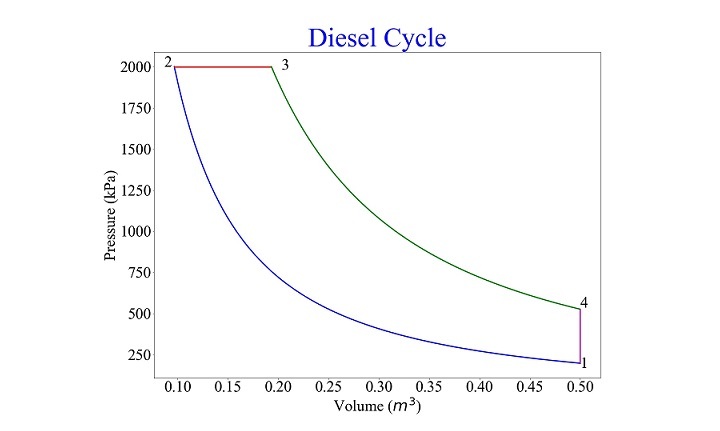
State |
p |
v |
|---|---|---|
1 |
2.000000e+05 |
0.500000 |
2 |
2.000000e+06 |
0.096535 |
3 |
2.000000e+06 |
0.193070 |
4 |
5.278032e+05 |
0.500000 |
Conclusion
In this tutorial, the Otto and Diesel cycles are modelled with the help of Python Programming. Function of Diesel and Otto cycles were Programmed and tested. The function is capable to draw the cycles based on the input data.

