
- Chemistry Notes for UPSC IAS Prelims (Part II)
- Chemistry - Home
- Chemistry - Introduction
- Chemistry - Branches
- Chemistry - Radioactivity
- Chemistry - Nuclear Energy
- Chemistry - Metals
- Chemistry - Metallurgy
- Chemistry - Sodium
- Chemistry - Calcium
- Chemistry - Aluminum
- Chemistry - Magnesium
- Chemistry - Maganese
- Chemistry - Iron
- Chemistry - Copper
- Chemistry - Silver
- Chemistry - Gold
- Chemistry - Platinum
- Chemistry - Zinc
- Chemistry - Mercury
- Chemistry - Plutonium
- Chemistry - Uranium
- Chemistry - Lead
- Chemistry - Thorium
- Chemistry - Hydrogen
- Chemistry - Helium
- Chemistry - Oxygen
- Chemistry - Carbon
- Chemistry - Nitrogen
- Chemistry - Chemical Law
- Chemistry - Discovery of Elements
- Elements With Their Valence
- Elements With Their Atomic Number
- Chemistry - Nobel Prize
Chemistry - Uranium
Introduction
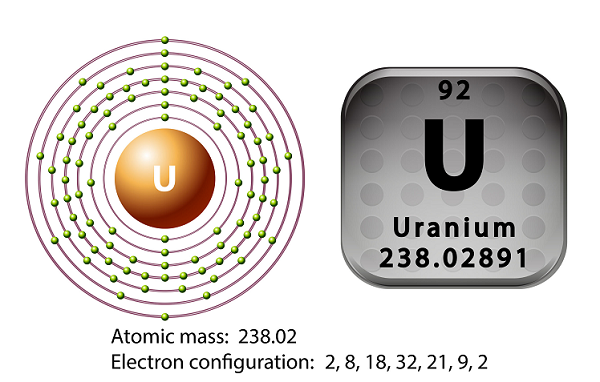
Uranium is the metal of the actinide series of the periodic table.
The symbol of uranium is ‘U’ and atomic number is ’92.’
In 1789, Martin Heinrich Klaproth had discovered the element uranium and named it after the name of Uranus.
Salient Features of Uranium
Uranium is a silvery-white metal.
A uranium atom has 92 electrons as well as 92 protons, of which 6 are valence electrons.
Because of having unstable isotopes, uranium is a weak radioactive element.
Uranium-238 is the most common isotope of uranium.
Uranium occurs naturally in very low concentrations i.e. a few parts per million in rock, soil, and water.
Uranium decays gradually (slowly) by emitting its alpha particle.
Uranium has poor electric conductivity (so poor conductor of electricity).
Uranium is malleable, ductile, and marginally paramagnetic
Occurrence of Uranium
Uranium is (naturally) found as uranium-238, uranium-235, and uranium-234.
The half-life of uranium-238 is about 4.47 billion years almost the age of the Earth and the half-life of uranium-235 is about 704 million years.
Alloys of Uranium
Following are the major alloys of Uranium −
Staballoy
Uranium hydride
Compounds of Uranium
Following are the major compounds of Uranium −
Uranium nitride - U2N3
Uranium pentafluoride - UF5
Uranium carbide - UC
Uranyl fluoride - UO2F2
Uranium dioxide - UO2
Uranium hexafluoride - UF6
Triuranium oxtoxide - U3O8
Uranium tetrafluoride - UF4
Uranium trioxide - UO3
Uranium tetrachloride - Ucl4
Uranyl nitrate - UO2(NO3)2
Uses of Uranium
Uranium is used as power source in nuclear submarines (especially by military).
Uranium is used in making nuclear weapons.
Uranium is also used as ballasts for ships.