
- Chemistry Notes for UPSC IAS Prelims (Part II)
- Chemistry - Home
- Chemistry - Introduction
- Chemistry - Branches
- Chemistry - Radioactivity
- Chemistry - Nuclear Energy
- Chemistry - Metals
- Chemistry - Metallurgy
- Chemistry - Sodium
- Chemistry - Calcium
- Chemistry - Aluminum
- Chemistry - Magnesium
- Chemistry - Maganese
- Chemistry - Iron
- Chemistry - Copper
- Chemistry - Silver
- Chemistry - Gold
- Chemistry - Platinum
- Chemistry - Zinc
- Chemistry - Mercury
- Chemistry - Plutonium
- Chemistry - Uranium
- Chemistry - Lead
- Chemistry - Thorium
- Chemistry - Hydrogen
- Chemistry - Helium
- Chemistry - Oxygen
- Chemistry - Carbon
- Chemistry - Nitrogen
- Chemistry - Chemical Law
- Chemistry - Discovery of Elements
- Elements With Their Valence
- Elements With Their Atomic Number
- Chemistry - Nobel Prize
Chemistry - Sodium
Introduction
Sodium is a soft, silvery color, and highly reactive alkali metal.
In the periodic table, Sodium is kept in group 1, as it has single electron in its outer shell.
The symbol of sodium is ‘Na,’ which has been actually taken from Latin word ‘natrium.’
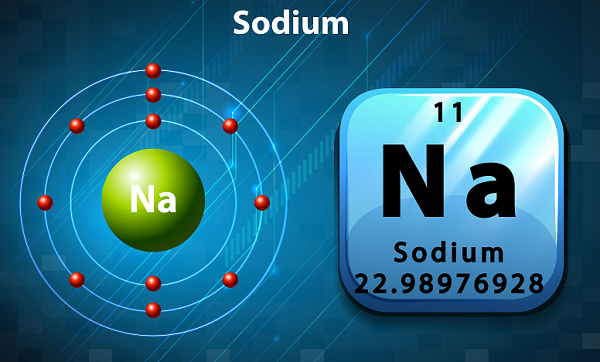
In terms of abundance, sodium is the sixth element found in the Earth's crust.
Sodium exists in various minerals including feldspars, sodalite, and rock salt (NaCl).
In 1807, Humphry Davy first isolated sodium by the electrolysis of sodium hydroxide.
By the time, 20 isotopes of sodium are known, but among all, only 23Na is stable.
Salient Features of Sodium
Following are the major features of sodium element −
Sodium metal a soft element that be can be easily cut with a knife.
Sodium is a good conductor of heat and electricity.
Because of having low atomic mass and large atomic radius, sodium is one of the least dense elements (third least dense element – first two are lithium and potassium).
Sodium can float on water.
Sodium along its compounds glow yellow (see image below).

Sodium compounds have very high commercial importance and have high demand in the industries of glass, paper, soap, and textiles.
Sodium Compounds
Following are some of the significant examples of sodium compounds −
Table salt -(NaCl)
Soda ash -(Na2CO3)
Baking soda -(NaHCO3)
Caustic soda -(NaOH)
Sodium nitrate -(NaNO3)
Sodium thiosulfate -(Na2S2O3·5H2O)
Borax -(Na2B4O7·10H2O)
Occurrence of Sodium
The crust of Earth contains about 2.27% sodium.
Sodium is the 5th most abundant metal; other four are aluminum, iron, calcium, and magnesium.
In the oceanic water, about 1.08 × 104 milligrams sodium found in per liter.
Sodium does not found as a pure element, as it is highly reactive.
Uses of Sodium
Following are the major uses of sodium −
Sodium chloride is highly useful for anti-icing and de-icing as well as a preservative.
In cooking, sodium bicarbonate is used.
Sodium and some of its compounds are used in medicines.
In comparison to potassium (which is a better ion), sodium is more frequently used because of its lower price and atomic weight.
In organic chemistry, sodium hydride is used as various reactions.
Metallic sodium is principally used for the production of sodium borohydride, sodium triphenylphosphine, azide, indigo, etc.
In some fast reactors, liquid sodium is used as a heat transfer fluid because of having the property of good heat conductivity.
Sodium is also an essential mineral for the human health, as it regulates blood pressure, blood volume, osmotic equilibrium, and pH value.
The minimum amount of 500 milligrams sodium is required every day for a healthy human body.