
- Chemistry Notes for UPSC IAS Prelims (Part II)
- Chemistry - Home
- Chemistry - Introduction
- Chemistry - Branches
- Chemistry - Radioactivity
- Chemistry - Nuclear Energy
- Chemistry - Metals
- Chemistry - Metallurgy
- Chemistry - Sodium
- Chemistry - Calcium
- Chemistry - Aluminum
- Chemistry - Magnesium
- Chemistry - Maganese
- Chemistry - Iron
- Chemistry - Copper
- Chemistry - Silver
- Chemistry - Gold
- Chemistry - Platinum
- Chemistry - Zinc
- Chemistry - Mercury
- Chemistry - Plutonium
- Chemistry - Uranium
- Chemistry - Lead
- Chemistry - Thorium
- Chemistry - Hydrogen
- Chemistry - Helium
- Chemistry - Oxygen
- Chemistry - Carbon
- Chemistry - Nitrogen
- Chemistry - Chemical Law
- Chemistry - Discovery of Elements
- Elements With Their Valence
- Elements With Their Atomic Number
- Chemistry - Nobel Prize
Chemistry - Introduction
Introduction
Chemistry is a branch of Natural Science that studies about the structure, composition, and changing properties of matters.
Chemistry studies the smallest part of a matter i.e. atom (along with its all properties) to the large materials (e.g. gold, silver, iron, etc.) and their properties.
Chemistry also studies the intermolecular forces (that provide matter the general properties) and the interactions between substances through the chemical reactions.

In 1998, Professor Raymond Chang defined Chemistry as −
"Chemistry" to mean the study of matter and the changes it undergoes.
It is believed that the study of chemistry started with the theory of four elements propounded by Aristotle.
The four theory of elements states that “fire, air, earth, and water were the fundamental elements from which everything is formed as combination.”
Because of his classical work namely “The Sceptical Chymist,” Robert Boyle, is known as the founding father of chemistry.
Boyle formulated a law, became popular as ‘Boyle’s Law.’
Boyle’s law is an experimental gas law that analyzes the relationship between the pressure of a gas and volume of the respective container.
By advocating his law, Boyle rejected the classical ‘four elements’ theory.
The American scientists Linus Pauling and Gilbert N. Lewis collectively propounded the electronic theory of chemical bonds and molecular orbitals.
The United Nations declared 2011 as the ‘International Year of Chemistry.’
The matter is defined in chemistry as anything that has rest mass and volume and also takes space.
The matter is made up of particles.
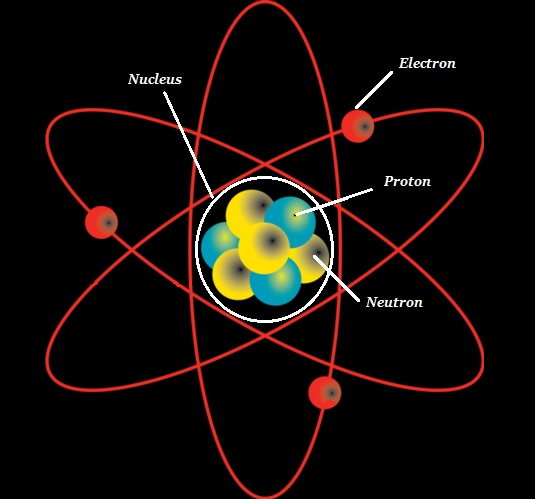
The atom is the fundamental unit of chemistry.
The atom consists of a dense core known as the atomic nucleus and it is surrounded by a space known as the electron cloud.
The nucleus (of an atom) is composed of protons (+ve charged particles) and neutrons (neutral or uncharged particles); collectively, these two are known as nucleons (as shown in the image given below).
A chemical element is a pure form of a substance; it consists of single type of atom.
The periodic table is the standardized representation of all the available chemical elements.
A compound is a pure form of a substance; it composed of more than one elements.
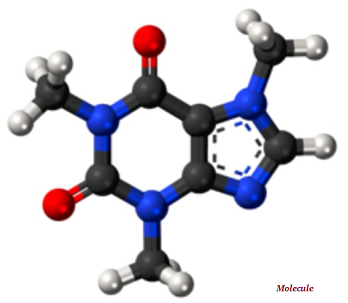
A molecule is the smallest indivisible part of a pure chemical substance; molecule has distinctive set of chemical properties (see the image given below).
To Continue Learning Please Login