
- Chemistry Notes for UPSC IAS Prelims (Part II)
- Chemistry - Home
- Chemistry - Introduction
- Chemistry - Branches
- Chemistry - Radioactivity
- Chemistry - Nuclear Energy
- Chemistry - Metals
- Chemistry - Metallurgy
- Chemistry - Sodium
- Chemistry - Calcium
- Chemistry - Aluminum
- Chemistry - Magnesium
- Chemistry - Maganese
- Chemistry - Iron
- Chemistry - Copper
- Chemistry - Silver
- Chemistry - Gold
- Chemistry - Platinum
- Chemistry - Zinc
- Chemistry - Mercury
- Chemistry - Plutonium
- Chemistry - Uranium
- Chemistry - Lead
- Chemistry - Thorium
- Chemistry - Hydrogen
- Chemistry - Helium
- Chemistry - Oxygen
- Chemistry - Carbon
- Chemistry - Nitrogen
- Chemistry - Chemical Law
- Chemistry - Discovery of Elements
- Elements With Their Valence
- Elements With Their Atomic Number
- Chemistry - Nobel Prize
Chemistry - Radioactivity
Introduction
The process of emission of particles from nuclei because of the nuclear instability; is known as radioactivity.
The substance that releases such energy/rays is known as radioactive substance.
The invisible rays released from such radioactive substance are known as radioactive rays.
Likewise, radioactivity is a nuclear phenomenon that happens (naturally) because of the nuclear instability of atoms.
In 1896 Henri Becquerel first observed the phenomena of radioactivity, but the term ‘radioactivity’ was coined by Marie Curie.
Marie Curie discovered the radioactive elements namely Polonium and Radium in 1898.
For her discovery, Marie Curie won the Nobel Prize.
Radioactive Rays
After long years of experiment, Ernest Rutherford along with his colleague (Hans Geiger and his student Ernest Marsden), discovered alpha rays, beta rays, and gamma rays.
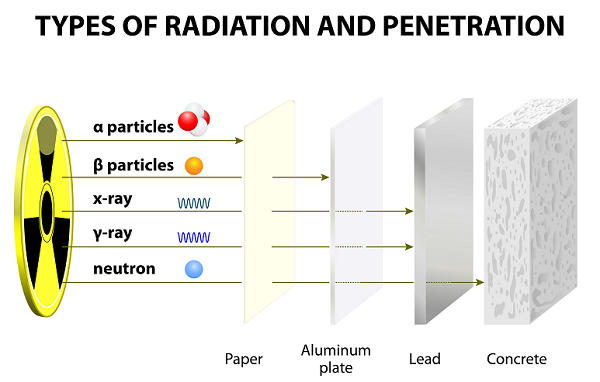
These rays emitted as the result of the disintegration of atoms.
Alpha (α) Particles
Alpha particles are usually composed of two protons and two neutrons, which are tightly bound together.
Alpha particles are being released during radioactive decay (or alpha decay) from the nucleus radio nuclides.
The alpha particles are identical to the nucleus of either normal helium atom or doubly ionized helium atom.
In comparison to other particles (i.e. Gamma and Beta), alpha particles are heavy and slow. Therefore, alpha particles have very small range in the air.
Because of slow speed, Alpha particles have very weak penetrating powers; these particles are even stopped by a thin paper sheet (see image given above).
Because of having the double positive charge, alpha particles are highly ionizing.
Beta (β) Particles
Beta particles are the fast moving electrons emitted by some radio nuclides during the radioactive decay (also known as beta decay).
Beta particles are of much lighter weight and carry a single negative charge.
Beta particles are rarely ionizing than the alpha particles.
Because of having lighter weight, beta particles can travel much farther than alpha particles; however, beta particles can be stopped by several sheet of papers or one sheet of aluminum.
Beta particles are negatively charged and get attracted towards positively charged particles.
Gamma (ү) Particles
Gamma particles are the bundle of high energy namely electromagnetic energy (photon) emitted by the radioactive elements during the radioactive decay.
Among all three particles (alpha, beta, and gamma), gamma particles are the most energetic photons.
Gamma particles, which are the form of electromagnetic radiation(EMR), originate from the nucleus.
The wavelengths of gamma are the shortest among all three.
Gamma particles have no charge and they are neutral; therefore, they are unaffected by magnetic and electric fields.
Uses of Radioactive Elements
Radioactive elements are used in −
Medical field (treatment of many diseases)
Industrial process
Energy production – Nuclear reactors
To Continue Learning Please Login