
 Data Structure
Data Structure Networking
Networking RDBMS
RDBMS Operating System
Operating System Java
Java MS Excel
MS Excel iOS
iOS HTML
HTML CSS
CSS Android
Android Python
Python C Programming
C Programming C++
C++ C#
C# MongoDB
MongoDB MySQL
MySQL Javascript
Javascript PHP
PHP
- Selected Reading
- UPSC IAS Exams Notes
- Developer's Best Practices
- Questions and Answers
- Effective Resume Writing
- HR Interview Questions
- Computer Glossary
- Who is Who
Ion Exchange Chromatography: Principle, Steps and Uses
Introduction
Ion exchange chromatography is a powerful technique for separating and purifying proteins, peptides, nucleic acids, and other charged biomolecules based on their net charge.
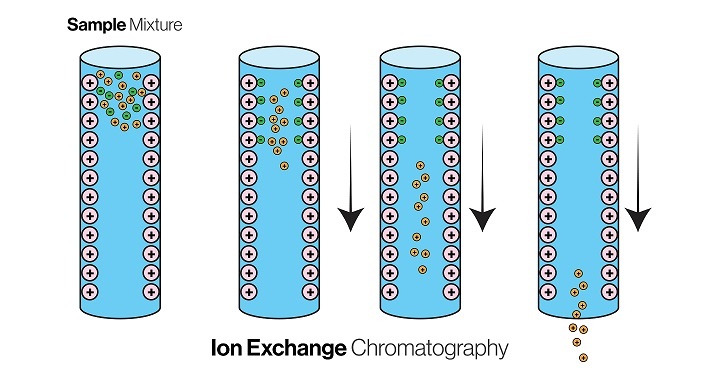
The technique involves the use of a stationary phase consisting of a resin with fixed charged groups that bind to the target molecules based on their charge characteristics. The principle of ion exchange chromatography is based on the differential affinity of biomolecules for the charged groups on the resin, which enables selective separation of the target molecule from a complex mixture of biomolecules.
The content below elaborates the principle, steps, and uses of ion exchange chromatography in detail.
Principle of Ion Exchange Chromatography
The principle of ion exchange chromatography is based on the electrostatic interaction between charged biomolecules and charged groups on the resin.
The stationary phase consists of a resin with fixed charged groups that are either positively or negatively charged. When a mixture of charged biomolecules is passed through the resin, the molecules with the opposite charge to that of the stationary phase are attracted to and bound to the resin, while the molecules with the same charge as the stationary phase are repelled and pass through the column unbound.
The bound molecules can then be eluted from the column by changing the ionic strength or pH of the elution buffer, which disrupts the electrostatic interaction between the charged groups on the resin and the bound biomolecules.
The two types of ion exchange chromatography are -
Anion exchange chromatography and
Cation exchange chromatography.
Anion exchange chromatography uses a positively charged resin to bind negatively charged biomolecules.
Cation exchange chromatography uses a negatively charged resin to bind positively charged biomolecules. The choice of resin and buffer conditions depends on the charge characteristics of the target biomolecule.
Steps in Ion Exchange Chromatography
Ion exchange chromatography involves several steps which are as follows,
Column Packing
The first step in ion exchange chromatography is column packing, which involves packing the resin into a chromatography column. The resin is typically packed into a pre-equilibrated column using a slurry of resin beads in an appropriate buffer.
The column is then washed with buffer to remove any unbound resin beads and to equilibrate the column with the desired buffer conditions.
Sample Loading
Once the column is equilibrated, the sample containing the target biomolecule is loaded onto the column. The sample is typically applied at a low flow rate to ensure even distribution of the sample on the resin bed.
The sample is loaded onto the column in a buffer that matches the equilibration buffer, which ensures that the target biomolecule remains in a stable state throughout the loading process.
Washing
After the sample is loaded onto the column, the column is washed with buffer to remove any unbound biomolecules and to reduce non-specific binding.
The washing step typically involves the use of a high salt concentration buffer that disrupts non-specific interactions between the biomolecules and the resin. The buffer is then switched back to the equilibration buffer to ensure that the target biomolecule remains bound to the resin.
Elution
The final step in ion exchange chromatography is elution, which involves the release of the bound biomolecule from the resin. Elution is typically achieved by changing the ionic strength or pH of the elution buffer, which disrupts the electrostatic interaction between the charged groups on the resin and the bound biomolecule.
The elution conditions must be carefully optimized to ensure that the target biomolecule is eluted from the column with high purity and yield.
Uses of Ion Exchange Chromatography
Some of the most common uses of ion exchange chromatography include ?
Protein Purification
Many proteins have a net charge at physiological pH, which makes them amenable to separation by ion exchange chromatography. Anion exchange chromatography is often used to purify positively charged proteins, while cation exchange chromatography is used to purify negatively charged proteins. The technique can be used to separate a target protein from other contaminants in a complex mixture, such as other proteins, nucleic acids, and lipids.
Peptide Purification
Ion exchange chromatography is also commonly used for the purification of peptides. Peptides are typically more difficult to purify than proteins due to their smaller size and more complex mixture of impurities. Ion exchange chromatography can be used to separate a target peptide from other contaminants based on its net charge.
Nucleic Acid Purification
Ion exchange chromatography can be used for the purification of nucleic acids, such as DNA and RNA. The technique relies on the charge characteristics of the nucleic acids to separate them from other contaminants in a complex mixture. Anion exchange chromatography is often used to purify DNA, while cation exchange chromatography is used to purify RNA.
Vaccine Production
Ion exchange chromatography is widely used in the production of vaccines. The technique can be used to purify viral particles from cell culture supernatants, such as influenza virus, measles virus, and adenovirus. The technique can also be used to purify recombinant proteins that are used as vaccine antigens, such as human papillomavirus virus-like particles

Quality Control
Ion exchange chromatography is an important tool for quality control in the biopharmaceutical industry. The technique can be used to analyse the purity and identity of protein and peptide drugs, such as monoclonal antibodies and insulin.
Ion exchange chromatography can also be used to analyse the charge heterogeneity of these drugs, which can affect their bioactivity and pharmacokinetics.
Conclusion
Ion exchange chromatography is a powerful technique for the separation and purification of biomolecules based on their net charge. The technique relies on the electrostatic interaction between charged groups on the resin and charged biomolecules in a sample.
Ion exchange chromatography has a wide range of applications in the biopharmaceutical industry, including protein and peptide purification, nucleic acid purification, vaccine production, and quality control.
The technique is often used in combination with other chromatography techniques to achieve higher purity and to provide a comprehensive characterization of the biomolecule of interest.

