
 Data Structure
Data Structure Networking
Networking RDBMS
RDBMS Operating System
Operating System Java
Java MS Excel
MS Excel iOS
iOS HTML
HTML CSS
CSS Android
Android Python
Python C Programming
C Programming C++
C++ C#
C# MongoDB
MongoDB MySQL
MySQL Javascript
Javascript PHP
PHP
- Selected Reading
- UPSC IAS Exams Notes
- Developer's Best Practices
- Questions and Answers
- Effective Resume Writing
- HR Interview Questions
- Computer Glossary
- Who is Who
What is Affinity Chromatography?
Introduction
Affinity chromatography is a powerful technique used in the field of biochemistry to separate and purify proteins, enzymes, and other biomolecules from a complex mixture of biological samples.
This technique relies on the specific interaction between a target molecule and a ligand that is immobilized on a chromatographic support. Affinity chromatography is widely used in the purification of biological molecules, and its success can be attributed to its high specificity, high yield, and ease of use.
Affinity chromatography used in isolation of biological molecules
Given below is an overview of the principles, techniques, and applications of affinity chromatography.
Principles of Affinity Chromatography
Affinity chromatography is based on the principle of selective binding of a target molecule to a specific ligand. The target molecule can be a protein, enzyme, or other biomolecule that is present in a complex mixture of biological samples.
The ligand is a molecule that has a high affinity for the target molecule and can specifically bind to it. The interaction between the ligand and the target molecule is highly specific and can be controlled by various factors such as pH, temperature, ionic strength, and the presence of specific ions.
The ligand is immobilized on a solid support, which is packed in a chromatography column. The sample containing the target molecule is passed through the column, and the target molecule binds to the ligand.
The other components of the sample, which do not bind to the ligand, are washed out of the column. The target molecule is then eluted from the column by changing the conditions of the column, such as pH or ionic strength, which disrupts the interaction between the ligand and the target molecule.
Types of Ligands
The success of affinity chromatography depends on the choice of the ligand. The ligand should have a high affinity for the target molecule, and it should be able to specifically bind to the target molecule.
There are various types of ligands that can be used in affinity chromatography, including ?
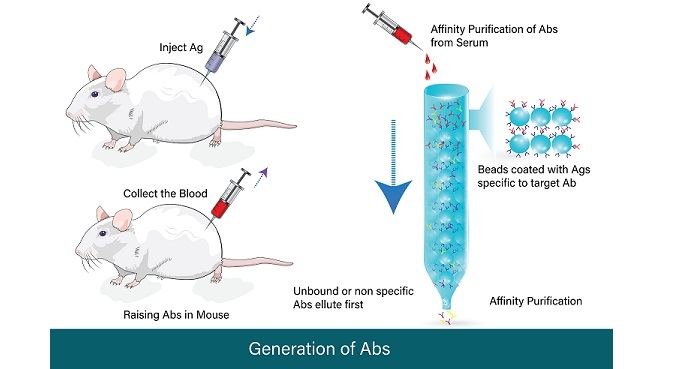
Antibodies
Antibodies are highly specific proteins that can specifically bind to their antigen. Antibodies can be used as ligands in affinity chromatography to purify antigens from biological samples.
Enzymes
Enzymes can also be used as ligands in affinity chromatography. Enzymes can specifically bind to their substrates or inhibitors, and this property can be used to purify enzymes from biological samples.
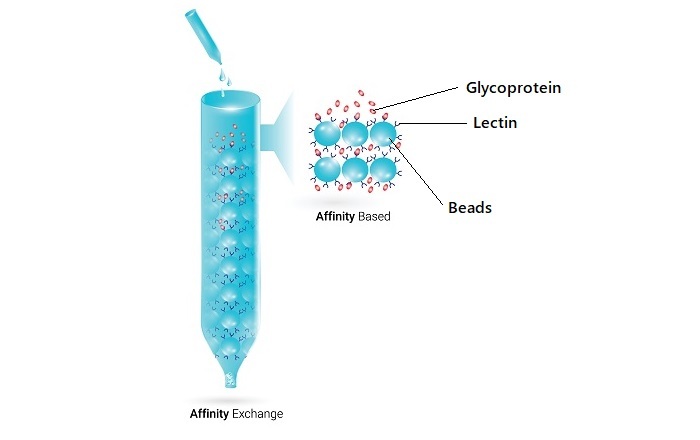
Lectins
Lectins are proteins that can specifically bind to carbohydrates. Lectins can be used as ligands in affinity chromatography to purify glycoproteins from biological samples.
Nucleic Acids
Nucleic acids such as DNA and RNA can also be used as ligands in affinity chromatography. They can specifically bind to proteins that have a high affinity for nucleic acids.
Ligands That Mimic Natural Ligands
Synthetic ligands that mimic the natural ligands of the target molecule can also be used in affinity chromatography. These ligands have a high affinity for the target molecule and can specifically bind to it.
Techniques of Affinity Chromatography
Some of the commonly used techniques are ?
Immobilized Metal Ion Affinity Chromatography (IMAC)
IMAC is a technique that uses metal ions such as nickel, cobalt, and copper as the ligands. The metal ions are immobilized on a chromatographic support, and they can specifically bind to histidine-tagged proteins. The histidine-tagged proteins can be eluted from the column by using a buffer containing imidazole.
Protein A/G Affinity Chromatography
Protein A/G affinity chromatography is a technique that uses protein A or G as the ligand. Protein A and G are bacterial proteins that can specifically bind to the Fc region of immunoglobulins. This technique is commonly used to purify antibodies from biological samples.
Lectin Affinity Chromatography
Lectin affinity chromatography is a technique that uses lectins as the ligand. Lectins can specifically bind to carbohydrates present on glycoproteins. This technique is commonly used to purify glycoproteins from biological samples.
Affinity Chromatography Using Affinity Tags
Affinity chromatography using affinity tags is a technique that uses small peptide sequences as affinity tags. The peptide sequences are attached to the target molecule, and they can specifically bind to the ligand immobilized on the chromatographic support. This technique is commonly used to purify recombinant proteins.
Applications of Affinity Chromatography
Affinity chromatography has a wide range of applications in the field of biochemistry. Some of the commonly used applications are ?
Purification of Proteins
Affinity chromatography is widely used in the purification of proteins from biological samples. Proteins can be purified based on their specific interactions with ligands that are immobilized on chromatographic supports.
Purification of Enzymes
Affinity chromatography can be used to purify enzymes from biological samples. Enzymes can be purified based on their specific interactions with ligands that are immobilized on chromatographic supports.
Purification of Antibodies
Affinity chromatography using protein A/G can be used to purify antibodies from biological samples. This technique is commonly used in the production of therapeutic antibodies.
Purification of Glycoproteins
Affinity chromatography using lectins can be used to purify glycoproteins from biological samples. This technique is commonly used in the analysis of glycoproteins.
Purification of Recombinant Proteins
Affinity chromatography using affinity tags can be used to purify recombinant proteins. This technique is commonly used in the production of recombinant proteins for research and therapeutic purposes.
Advantages and Limitations of Affinity Chromatography
Advantages of Affinity Chromatography
High Specificity: Affinity chromatography has a high specificity for the target molecule, which allows for the purification of the target molecule with a high degree of purity.
High Yield: Affinity chromatography has a high yield, which means that a large amount of the target molecule can be purified from a small amount of biological sample.
Easy to Use: Affinity chromatography is relatively easy to use and does not require complex equipment.
Limitations of Affinity Chromatography
Cost: Affinity chromatography can be expensive due to the cost of the ligands and chromatographic supports.
Low Binding Capacity: Affinity chromatography has a low binding capacity, which means that only a small amount of the target molecule can be purified at a time.
Non-Specific Binding: Affinity chromatography can suffer from non-specific binding, which can result in the purification of unwanted proteins.
Conclusion
Affinity chromatography is a powerful technique used in the field of biochemistry to separate and purify proteins, enzymes, and other biomolecules from a complex mixture of biological samples.
This technique relies on the specific interaction between a target molecule and a ligand that is immobilized on a chromatographic support.
Affinity chromatography has a wide range of applications in the field of biochemistry and is widely used in the purification of biological molecules due to its high specificity, high yield, and ease of use. However, it is important to consider the advantages and limitations of affinity chromatography.

