
 Data Structure
Data Structure Networking
Networking RDBMS
RDBMS Operating System
Operating System Java
Java MS Excel
MS Excel iOS
iOS HTML
HTML CSS
CSS Android
Android Python
Python C Programming
C Programming C++
C++ C#
C# MongoDB
MongoDB MySQL
MySQL Javascript
Javascript PHP
PHP
- Selected Reading
- UPSC IAS Exams Notes
- Developer's Best Practices
- Questions and Answers
- Effective Resume Writing
- HR Interview Questions
- Computer Glossary
- Who is Who
Column Chromatography: Principle, Types and Significance
Introduction
Column chromatography is one of the most versatile and widely used techniques for separating and purifying mixtures of organic compounds. It is a type of liquid chromatography where the stationary phase is a solid adsorbent material packed into a column, and the mobile phase is a liquid or gas that flows through the column.

This technique is based on the principles of adsorption, partition, and ion exchange, and it can be used to separate a wide range of compounds, including small molecules, proteins, and nucleic acids. In this article, we will discuss the principle, types, and significance of column chromatography.
Principle of Column Chromatography
Column chromatography works on the principle of differential adsorption of the components of a mixture onto a solid adsorbent material. The solid adsorbent material is packed into a column, and the mixture is then introduced into the column.
The mobile phase, which can be a liquid or gas, is then passed through the column, causing the different components of the mixture to migrate through the column at different rates.

The components that are more strongly adsorbed onto the solid adsorbent material will migrate more slowly through the column, while the components that are less strongly adsorbed will migrate more quickly.
The rate of migration of a particular component depends on its physicochemical properties, such as its polarity, size, and shape. Components that are more polar will be more strongly adsorbed onto the solid adsorbent material and will migrate more slowly through the column. On the other hand, components that are less polar will be less strongly adsorbed and will migrate more quickly through the column.
Types of Column Chromatography
There are several types of column chromatography, each of which is based on different principles of separation. The most common types of column chromatography are ?
Adsorption Chromatography
Adsorption chromatography is the most commonly used type of column chromatography. It works on the principle of differential adsorption of the components of a mixture onto a solid adsorbent material.
The stationary phase is a solid adsorbent material, such as silica gel, alumina, or cellulose, which is packed into a column. The mobile phase is a liquid or gas that flows through the column.
The components of the mixture are separated based on their different affinities for the solid adsorbent material.
Ion Exchange Chromatography
Ion exchange chromatography is used for the separation of charged compounds, such as proteins and nucleic acids. It works on the principle of differential binding of the charged components of a mixture to an ion exchange resin.
The stationary phase is an ion exchange resin, which contains either positively or negatively charged groups. The mobile phase is a buffer solution of the appropriate pH, which is used to control the ionic strength of the solution. The charged components of the mixture are separated based on their different affinities for the ion exchange resin.
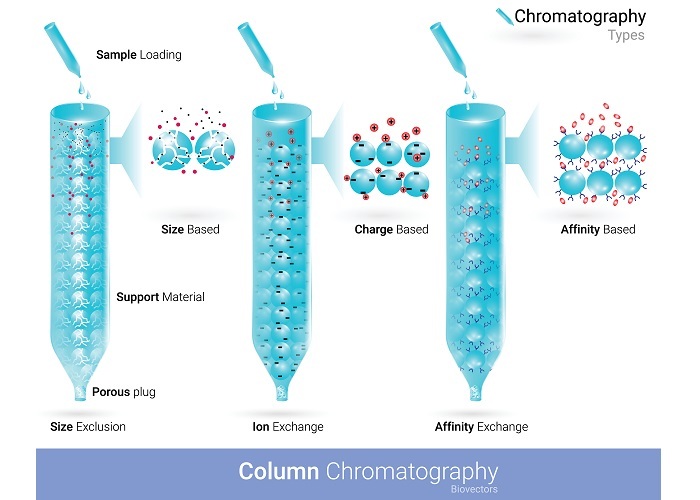
Size Exclusion Chromatography
Size exclusion chromatography is used for the separation of molecules based on their size. It works on the principle of differential exclusion of the components of a mixture based on their size.
The stationary phase is a gel matrix that contains pores of different sizes. The mobile phase is a liquid that flows through the column. The larger molecules are excluded from the pores and pass through the column more quickly, while the smaller molecules are trapped in the pores and pass through the column more slowly.
Affinity Chromatography
Affinity chromatography is used for the separation of biomolecules, such as enzymes and antibodies, based on their specific interactions with ligands. It works on the principle of differential binding of the components of a mixture to a ligand that is immobilized on a solid support. The stationary phase is a ligand that is immobilized on a solid support, such as a resin or a membrane. The mobile phase is a buffer solution that is used to wash and elute the bound biomolecules.
The biomolecules of interest are selectively bound to the ligand, while other unwanted components are washed away. The bound biomolecules are then eluted from the stationary phase using a buffer solution with a specific pH, ionic strength, or concentration of a competing ligand.
Significance of Column Chromatography
Column chromatography is a powerful technique for separating and purifying complex mixtures of organic compounds. It has a wide range of applications in the fields of chemistry, biochemistry, and biotechnology.
Drug Discovery
Column chromatography is used extensively in the field of drug discovery to purify and isolate active pharmaceutical ingredients (APIs) from natural products or synthetic compounds. It is often used in combination with other analytical techniques, such as high-performance liquid chromatography (HPLC), mass spectrometry (MS), and nuclear magnetic resonance (NMR) spectroscopy, to identify and characterize the purified compounds.
Protein Purification
Column chromatography is also widely used in the field of biochemistry to purify proteins and other biomolecules. It can be used to separate proteins based on their size, charge, hydrophobicity, or specific binding properties. For example, ion exchange chromatography can be used to separate proteins based on their net charge, while size exclusion chromatography can be used to separate proteins based on their size.
DNA Sequencing
Column chromatography is also used in DNA sequencing to purify and separate DNA fragments based on their size. Gel electrophoresis is a type of column chromatography that is used to separate DNA fragments based on their charge and size.
The DNA fragments are loaded onto a gel matrix and subjected to an electric field. The smaller fragments migrate more quickly through the gel matrix and are separated from the larger fragments.
Conclusion
In conclusion, column chromatography is a powerful technique for separating and purifying complex mixtures of organic compounds. It is based on the principles of adsorption, partition, and ion exchange and can be used to separate a wide range of compounds, including small molecules, proteins, and nucleic acids. There are several types of column chromatography, each of which is based on different principles of separation.
The most common types are adsorption chromatography, ion exchange chromatography, size exclusion chromatography, and affinity chromatography.

