
 Data Structure
Data Structure Networking
Networking RDBMS
RDBMS Operating System
Operating System Java
Java MS Excel
MS Excel iOS
iOS HTML
HTML CSS
CSS Android
Android Python
Python C Programming
C Programming C++
C++ C#
C# MongoDB
MongoDB MySQL
MySQL Javascript
Javascript PHP
PHP
- Selected Reading
- UPSC IAS Exams Notes
- Developer's Best Practices
- Questions and Answers
- Effective Resume Writing
- HR Interview Questions
- Computer Glossary
- Who is Who
Protein Targeting or Protein Sorting
Protein Targeting
The biological process by which proteins are delivered to their proper locations inside or outside the cell is known as protein targeting or protein sorting. Proteins can be secreted to the outside of the cell, the plasma membrane, various intracellular membranes, the interior of an organelle, or the plasma membrane.
The protein itself contains information that controls the delivery mechanism. Sorting correctly is essential for the cell, and problems with sorting have been connected to a number of illnesses.
Introduction
In eukaryotic cells, various proteins must be delivered to various locations within the cell or, in some situations, exported beyond the cell into the extracellular environment. How do the proper proteins reach the appropriate locations?
Proteins are delivered to their intended locations through a variety of shipping mechanisms that are present in cells, acting somewhat like a molecular postal service. In these systems, proteins are "addressed" for delivery to certain places using molecular labels, frequently amino acid sequences. Let's examine how these delivery systems function.
Signal Peptides
By acting as targeting signals, signal peptides allow the cellular transport system to route proteins to particular intracellular or extracellular regions. Although no consensus sequence for signal peptides has been found, many of them nevertheless have a distinctive tripartite structure ?
A hydrophilic, positively charged area close to the N-terminal.
A section of the signal peptide between 10 and 15 hydrophobic amino acids.
A somewhat polar area closes to the C-terminal that favors amino acids with shorter side chains when they are located close to the cleavage point.
The signal peptide is typically cut by a signal peptidase once a protein has reached its destination.
As a result, signal peptides are absent from the majority of mature proteins. While the targeting sequence is present on the C-terminal extension of peroxisomes, the majority of signal peptides are found at the N-terminal.
Protein Translocation
Proteins intended for secretion or a particular organelle must be transported because the cytosol is where a ribosome translates mRNA into protein. Co-translational translocation is the term for this process, which can also take place during translation, or post-translational translocation, which occurs after translation is finished.
Targeting of Proteins
Mitochondria
The majority of mitochondrial proteins are created as cytosolic precursors with uptake peptide signals, while certain proteins in the organelle are made from mitochondrial DNA.
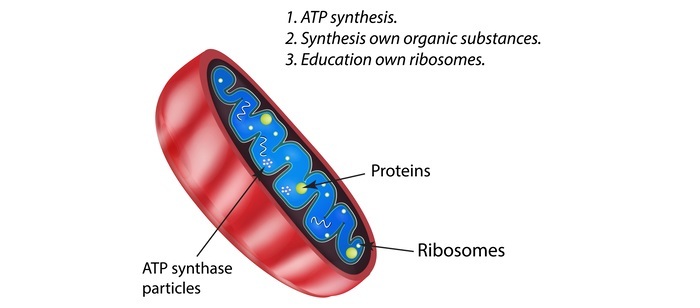
Depending on their sequences, unfolded proteins bound by the cytosolic chaperone hsp70 and sent to the mitochondria may be localized to four different locations. They could be directed at the inner membrane, intermembrane gap, outer membrane, or mitochondrial matrix. Health and disease have been related to flaws in one or more of these mechanisms.
Mitochondrial Matrix
The signal sequence is cut by a processing peptidase when the polypeptide reaches the matrix, and the remaining sequences are held by mitochondrial chaperones while they wait for appropriate folding and action.
An electrochemical gradient created by the mitochondrion during oxidative phosphorylation is what pushes and pulls the polypeptide from the cytosol to the intermembrane gap and then the matrix.
The intermembrane space has a positive potential and the matrix has a negative potential as a result of a mitochondrion that is actively engaged in metabolism. The positively charged portions of the targeting sequence are guided to their intended place by the matrix's negative potential.
Mitochondrial Inner Membrane
Depending on their overall sequences, mitochondrial proteins can be directed to the inner membrane by one of three alternative pathways, although they can still enter from the outer membrane utilizing the TOM20/22 import receptor complex and the TOM40 general import core.
In the first pathway for proteins targeted to the inner membrane, which has a matrix targeting sequence that directs the polypeptide to the inner membrane complex containing the previously stated translocase complex, the stages are the same as those for proteins targeted to the matrix.
Mitochondrial Outer Membrane
Using internal-targeting sequences to create hydrophobic alpha helices or beta barrels that span the phospholipid bilayer, precursor proteins interact with outer membrane translocase complexes to embed themselves into the membrane. This process is known as outer membrane targeting.
Depending on the intrinsic sequences of the preprotein, this may happen via two alternative paths. The preprotein will use the mitochondrial import complex and be transported laterally to the membrane if it has internal hydrophobic areas capable of building alpha helices.
Preproteins that correlate to beta-barrel building proteins and have hydrophobic internal sequences will be imported from the aforementioned outer membrane complex TOM20/22 to the intermembrane gap.
As they engage with the TIM9/10 intermembrane-space protein complex, they are transferred to the outer membrane's sorting and assembly machinery (SAM), which lateralizes the targeted protein as a beta-barrel.
Chloroplasts
Depending on their sequence, proteins may be sent to the stroma, thylakoid lumen, outer envelope, inner envelope, or thylakoid membrane of the chloroplast.
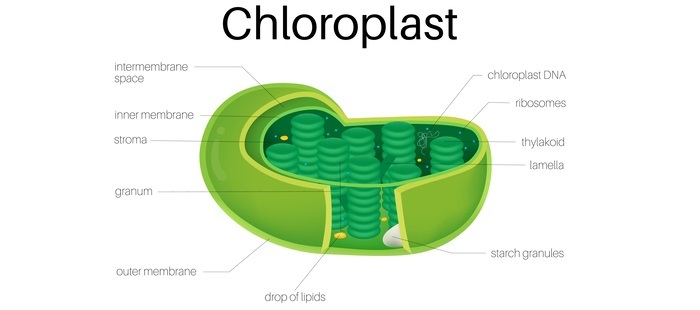
Because they typically lack a cleavable sorting sequence, proteins that are directed to the chloroplast envelope are laterally displaced by membrane sorting complexes.
The Toc and Tic complexes inside the chloroplast envelope are required for the general import of the bulk of preproteins from the cytosol.
The translocase of the outer chloroplast envelope is referred to as Toc, and the translocase of the inner chloroplast envelope is referred to as Tic. The Toc complex's function is made up of a minimum of three proteins.
Stroma
At least five different Tic proteins must be present in the Tic complex in order to create the translocation channel through the inner envelope. The stromal import sequence is cut off by a signal peptidase after it has reached the stroma.
According to current knowledge, ATP hydrolysis via stromal HSP chaperones drives this protein transport mechanism to the stroma rather than the transmembrane electrochemical gradient that is created in mitochondria to drive protein import.
Conclusion
The dual-targeting peptide generally has a character that is between the two specific ones. These proteins' targeting peptides contain relatively few negatively charged amino acids and a lot of basic and hydrophobic amino acids.
They contain less alanine and more leucine and phenylalanine than other foods. Compared to both mitochondrial and chloroplastic proteins, the dual targeted proteins feature a targeting peptide that is more hydrophobic. To determine a peptide's dual targeting status based solely on its physio-chemical properties is laborious.

