
 Data Structure
Data Structure Networking
Networking RDBMS
RDBMS Operating System
Operating System Java
Java MS Excel
MS Excel iOS
iOS HTML
HTML CSS
CSS Android
Android Python
Python C Programming
C Programming C++
C++ C#
C# MongoDB
MongoDB MySQL
MySQL Javascript
Javascript PHP
PHP
- Selected Reading
- UPSC IAS Exams Notes
- Developer's Best Practices
- Questions and Answers
- Effective Resume Writing
- HR Interview Questions
- Computer Glossary
- Who is Who
Signal Transduction: Definition and Pathways
Introduction
A cellular response produced as a result of a series of molecular processes, most frequently protein phosphorylation catalyzed by protein kinases, known as signal transduction, which is the process by which a chemical or physical signal is transferred through a cell. Although in some instances the term sensor is used, generally speaking, proteins that detect stimuli are referred to as receptors.
Definition
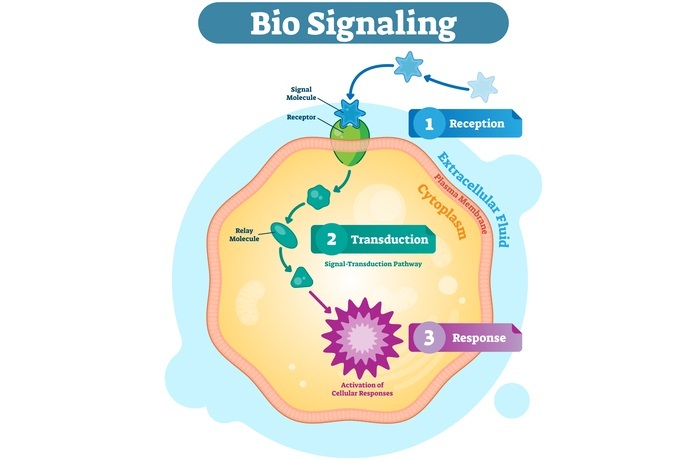
The process through which a cell reacts to things outside of it via signaling molecule that is both inside and on its surface. The majority of molecules that result in signal transduction are chemical substances that bind to a particular protein receptor (signaling molecule) on or in a cell, including hormones, neurotransmitters, and growth factors. The signals are then transmitted inside the cell from one molecule to another, which causes a certain cell reaction, such as cell division or cell death. Cells need to transmit signals in order to develop and function appropriately.
A signaling route, which is a series of biochemical events known as a biochemical cascade, is initiated by the changes brought about by ligand binding (or signal detecting) at a receptor. Signaling pathways connect with one another to build networks that enable the coordination of cellular responses, frequently through combinatorial signaling events.
Such reactions include modifications to gene transcription or translation, post-translational modifications to proteins' structure, and modifications to their localization at the molecular level.
The fundamental mechanisms governing cell growth, proliferation, metabolism, and many other functions are these molecular occurrences. Signal transduction pathways control cell communication in multicellular organisms in a wide range of ways. Each element of a signaling pathway is categorized based on the function it performs in relation to the initial stimulus.
First messengers are ligands, and signal transducers are receptors, which in turn activate primary effectors. These effectors, which are primarily proteins, are frequently connected to second messengers, which in turn can activate further effectors.
Signal gain is the idea that a signal can be amplified so that one signaling molecule can cause a reaction involving hundreds to millions of molecules depending on how effective the nodes are.
Similar to other signal transduction, biological signal transduction is characterized by lag, noise, signal feedback and feedforward, and interference, which can be minimal or severe.
The development of computational biology has made it possible to analyze signaling pathways and networks, particularly the signaling rewiring mechanisms underpinning reactions to acquired drug resistance, to better understand cellular functioning and disease.
Signal Transduction Pathways
The key signalling pathways that show how ligands interacting with their receptors can change second messengers and ultimately influence cellular responses are listed below.
MAPK/ERK pathway-
A system that connects intracellular reactions with growth factor binding to cell surface receptors, this pathway has a large number of protein constituents and is quite complicated. This mechanism is activated in many different cell types to stimulate cell proliferation, and many types of cancer are linked to abnormalities in this system.
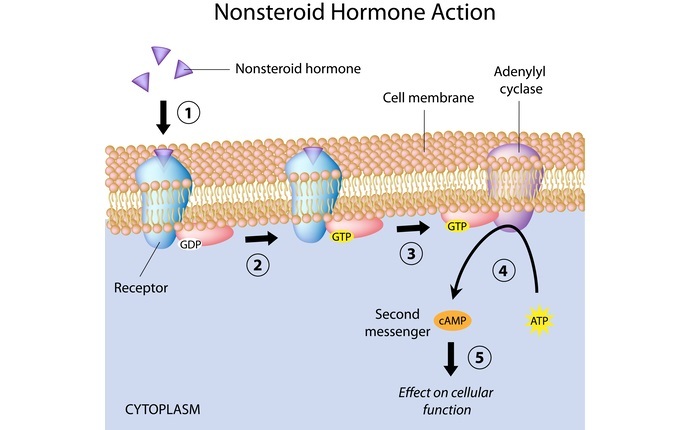
cAMP dependent pathway-
PKA, also known as cAMP-dependent protein kinase, is activated by cAMP in humans (see image), and as a result, subsequent consequences are mostly dependent on PKA, which varies depending on the kind of cell.
IP3/DAG Pathway-
PLC breaks down the phospholipid phosphatidylinositol 4,5-bisphosphate (PIP2) to produce inositol 1,4,5-triphosphate and diacyl glycerol (IP3). While IP3 is released as a soluble structure into the cytosol, DAG is still attached to the membrane.
The endoplasmic reticulum's IP3 receptors, which are specific calcium channels, are then bound by IP3 as it diffuses through the cytoplasm.
Only calcium can travel through these channels because they are solely available to it. As a result, the amount of calcium in the cytosol rises, resulting in a series of intracellular alterations and activities.
Moreover, calcium and DAG interact together to activate PKC, which then causes other molecules to be phosphorylated and alters cellular activity. Taste, manic depression, and tumor promotion are side effects.
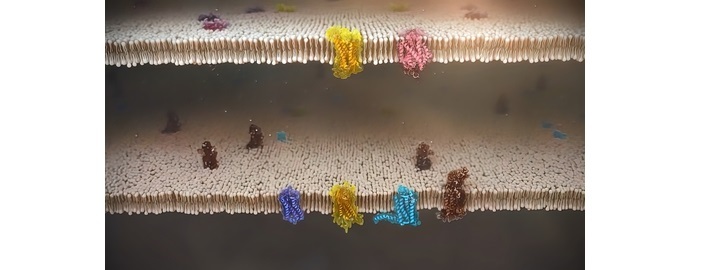
Extracellular Receptors
Tyrosine kinase and phosphatases are two examples of intracellular proteins that have an enzymatic activity in eukaryotic cells when they are triggered by a ligand/receptor interaction.
Such enzymes frequently have a covalent bond with the receptor. Some of them produce second messengers such cyclic AMP and IP3, the latter of which regulates the release of calcium reserves from intracellular reservoirs into cytoplasm.
In order to coordinate the signalling complexes and facilitate contacts between signalling proteins, other activated proteins engage in interactions with adaptor proteins. Both adaptor proteins and enzymes respond to different second messenger molecules.
Activated adaptor proteins and enzymes frequently have specialised protein domains that bind to particular secondary messenger molecules. For instance, the EF hand domains of calmodulin bind calcium ions, enabling it to bind and activate calmodulin-dependent kinase. The Pleckstrin homology domains of proteins, including the kinase protein AKT, are similarly affected by PIP3 and other phosphoinositides.
Intracellular Receptors
Nuclear receptors and cytoplasmic receptors, for example, are soluble proteins that are restricted to specific regions of the cell. Non-polar hormones like the steroid hormones testosterone and progesterone and derivatives of the vitamins A and D are the typical ligands for nuclear receptors.
The ligand must passively diffuse through the plasma membrane in order to start signal transduction. The ligands enter the nucleus through the nuclear membrane after interacting with the receptor, changing how genes are expressed.
Conclusion
Much theoretical (mathematical) advancement was made as a result of these insights. The first of them was a straightforward theory put forth by Bell that explained an apparent paradox.
While B cell-secreted antibodies' affinities rise as the immune response develops, clustering generates stable networks, making binding largely irreversible. DeLisi and Perelson created a theory to explain the dynamics of cell surface clustering on lymphocyte membranes and discovered how the affinity and valence of the ligand affected the size distribution of clusters over time.

