
- Chemistry - Home
- Matter In Our Surroundings
- Is Matter Around Us Pure
- Chemistry - Atoms & Molecules
- Chemistry - Structure Of The Atom
- Chemical Reactions and Equations
- Chemistry - Acids, Bases, and Salts
- Materials: Metals and Non-Metals I
- Chemistry - Metals & Non-Metals II
- Carbon & its Compounds
- Periodic Classification Of Elements
- Synthetic Fibres and Plastics
- Chemistry - Coal And Petroleum
- Chemistry - Combustion And Flame
Chemistry Part 1 - Quick Guide
Chemistry - Matter In Our Surroundings
Introduction
Everything found in this universe is made up of some materials, scientists have named them as matter. For example, the food we eat, the air we breathe, stones, clouds, stars, plants, animals, water, dust, everything is categorized as matter.
Characteristics of Particles of Matter
Particles of matter are very small, normally, not visible from naked eye.
Particles of matter keep moving continuously, which is known as the kinetic energy.
Kinetic energy of particles directly depends on the temperature, as temperature increases, the speed of the movement also increases.
The particles of matter have attracting force; therefore, they attract each other.
The attracting force of the particles keeps the particles together; however, the strength of the attracting force varies from one kind of matter to another.
States of Matter
Matter has three following states −
Solid State
Liquid State
Gaseous State
Lets discuss them in brief −
Solid State
All the solid materials have a definite shape, distinct boundaries, and fixed volumes.
Most of the solid materials have negligible compressibility.
All the solid materials have a natural tendency to maintain their shape when subjected to outside force.
The solid materials can be broken under applied force, but it is very difficult to change their shape, as they are rigid.
Liquid State
Unlike solids, liquids have no fixed shape; however, they have a fixed volume.
Liquids take up the shape of the container in which they are kept.
Liquids have the property to flow and change shape.
Gaseous State
Matter in the form of air, which is neither solid nor liquid, is known as gas. For example, oxygen, nitrogen, hydrogen, etc.
Unlike solid, gas has not definite size and shape.
The gases, such as liquefied petroleum gas (LPG used in cooking); compressed natural gas (CNG used as fuel in vehicles), etc. have high compressibility; therefore, large volume of a gas can be compressed into a small cylinder and can be transported easily.
Gases, normally, show the property of diffusing very fast into other gases. This is the reason that we can smell (either good or bad) from the distance.
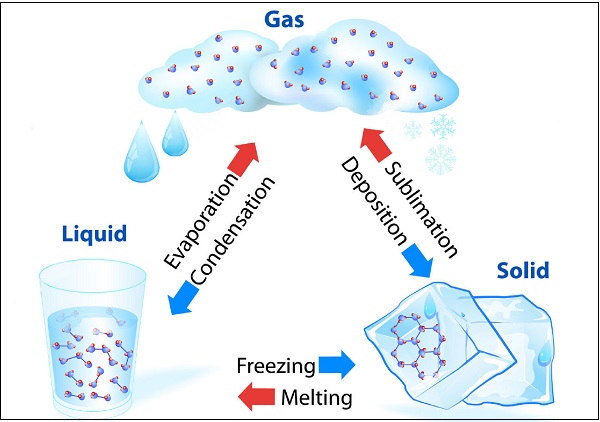
Matter Can Change its State
Water can exist in all three states, e.g. Ice as solid; water (H2O) as liquid; and water vapor as gas. The following diagram illustrates the transformation of water in different states −
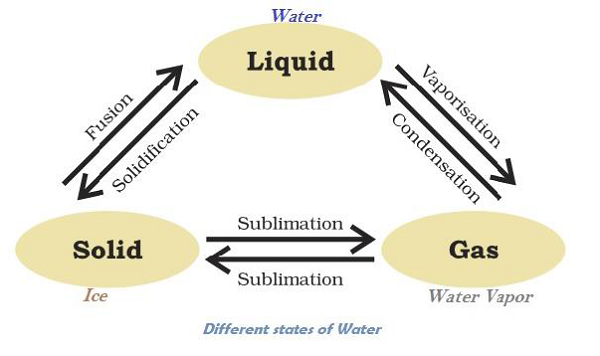
The temperature, at which solid melt and transform into the liquid (at the given atmospheric pressure), is known as melting point.
The melting point of a solid is an indication of the strength of the force of attraction between its particles.
The melting point of ice is 273.16 K, i.e. 00 C.
The process of melting (i.e. change of solid state into liquid state) is known as fusion.
The amount of heat energy, which is required to change 1 kg of a solid materials into liquid materials at a given atmospheric pressure, is known as the latent heat of fusion.
The temperature at which a liquid starts boiling at the given atmospheric pressure is known as boiling point.
The boiling point of water is 373 K i.e. 1000C.
A change of state of a matter directly from solid to gas without changing into liquid state (or vice versa) is known as sublimation.
The phenomenon i.e. change of a liquid into vapors at any temperature below its boiling point is known as evaporation.
Solid carbon dioxide (CO2) is stored under high pressure.

Solid CO2 gets converted directly into gaseous state once the pressure decreases to 1 atmosphere.
Atmosphere (atm) is a unit of measuring pressure exerted by the gas and the unit of pressure is Pascal (Pa); 1 atmosphere = 1.01 105 Pa.
The Fourth State of Matter
Plasma is the state that consists of super energetic and super excited particles.
The super excited particles are found in the form of ionized gases. E.g. the fluorescent tube (which contains helium gas) and neon sign bulbs (which contain neon gas) consist of plasma.
Chemistry - Is Matter Around Us Pure
Introduction
A pure substance is that that consists of single type of particle or particles.

Mixtures of two or more pure components without any undesirable substance are known as Mixtures, for example, water, minerals, soil etc.
A homogeneous mixture of two or more substances is known as solution. For example, lemonade, soda water etc.
Solution could be in any form such as it could be in liquid, solid, or gaseous.
Alloys are another example of mixture that contain homogeneous mixtures of metals; they cannot be separated into their components by physical methods. E.g. For example, brass is a mixture of zinc (approximately 30%) and copper (about 70%).
Significant Features of Solution
Solution is normally a homogeneous mixture.
The particles of a solution are even smaller than 1 nm (10-9 meter) in diameter and hence, these are not visible from the naked eyes.
The path of light is not visible in a solution.
The dissolved particles cannot be separated from the mixture by the simple process of filtration.
The dissolved particles do not settle down when it left undisturbed.
At a given temperature, when no more solute can be dissolved in a solution, it is known as saturated solution.
At a given temperature, the amount of the dissolved particles present in the saturated solution, is known as solubility.
Suspension
A suspension is a heterogeneous mixture in which the solute particles do not dissolve, but rather remain suspended throughout the bulk of the medium, is known as suspension.
Significant features of Suspension
Particles of a suspension are clearly visible from the naked eye.

The particles of a suspension scatter a beam of light that passes through it and likewise, its path is visible.
The salute particles can be separated from the mixture by the simple process of filtration.
Colloid
A heterogeneous mixture is known as colloid. E.g. mist, fog, smoke, face cream, etc.

The size of colloid particles is too small to see from the naked eye.
Colloid particles are big enough to scatter a beam of light passing through it and make the path visible.
Colloid particles cannot be separated from the mixture by the simple process of filtration.
The special filtration technique i.e. centrifugation, can be used to separate the colloidal particles.
Chromatography
The process of separation of components of a mixture is called as chromatography; normally it is used for the color separation.
Chromatography technique is used for separation of those solutes that dissolve in the same solvent.
Distillation
The process of purifying a liquid by heating and cooling means is known as distillation.
Crystallization
The process that separates a pure solid in the form of its crystals from a solution is known as crystallization.
Elements
In 1661, Robert Boyle was the first scientist who used the term element; Antoine Laurent Lavoisier, a French chemist, was the first who experimentally define the term element.
Element is as a basic form of matter that cannot be broken down into simpler substances by a chemical reaction.
Elements normally can be categorized as metals, non-metals, and metalloids.
Metal
A solid material, which typically is hard, ductile, malleable, shiny, and fusible with good electrical and thermal conductivity, is known as metal. E.g. gold, silver, copper, aluminum, etc.

Mercury is the only metal that remains liquid at room temperature.
Non-metal
All elements or substances, which are not metals, are known as non-metals. E.g. hydrogen, oxygen, iodine, carbon, etc.
Non-metals have variety of colors and they are poor conductors of heat and electricity.
Non-metals are not lustrous, sonorous, or malleable.
Compound
A substance, composed of two or more elements, is known as compound.
Compound is the result of the chemically combination of two or more elements in a fixed proportion.
Properties of a compound are somehow different from its constituent elements, whereas, the properties of a mixture are the same as of its constituting elements or compounds.
Chemistry - Atoms & Molecules
Introduction
Around 500 BC, an Indian Philosopher Maharishi Kanad, first time postulated the concept of indivisible part of matter and named it pramanu.
In 1808, John Dalton used the term atom and postulated the atomic theory to the study of matter.

Daltons Atomic Theory
According to Daltons atomic theory, all matter, whether an element, a compound or a mixture is composed of small particles called atoms.
According to Daltons atomic theory, all matters, whether they are elements, compounds, or mixtures, are composed of small particles known as atoms.
Salient features of Daltons Atomic Theory
All matter is made of very miniscule particles known as atoms.
Atom is an indivisible particle, which cannot be created or destroyed through chemical reaction.
All atoms of an element are identical in mass and chemical properties whereas, atoms of different elements have different masses and chemical properties.
To form a compound, atoms are combined in the ratio of small whole numbers.
In a given compound, the relative number and kinds of atoms are constant.
Atomic Mass
The mass of an atom of a chemical element; it is expressed in atomic mass units (symbol is u).
The atomic mass is roughly equivalent to the number of protons and neutrons present in the atom.
One atomic mass unit is a mass unit equal to the exactly one-twelfth (1/12th) the mass of one atom of carbon-12 and the relative atomic masses of all elements have been calculated with respect to an atom of carbon-12.
Molecule
The smallest particle of an element or a compound, which is capable to exist independently and shows all the properties of the respective substance.
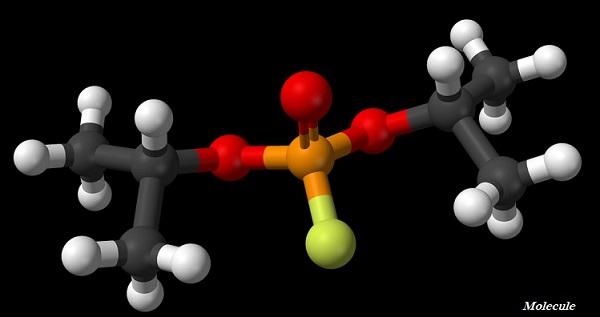
A molecule, normally, is a group of two or more atoms which are chemically bonded together.
Atoms of the same element or of different elements can join (with chemical bond) together to form molecules.
The number of atoms that constitute a molecule is known as its atomicity.
Ion
A charged particle is known as ion; it could be either negative charge or positive charge.
The positively charged ion is known as a cation.
The negatively charged ion is known as an anion.
Chemical Formulae
A chemical formula of a compound demonstrations its constituent elements and the number of atoms of each combining element.
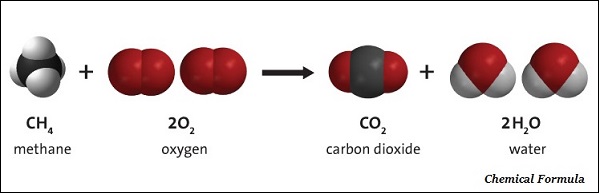
The chemical formula of a compound is the symbolic representation of its Composition.
The combining capacity of an element is known as its valency.
Molecular Mass
The molecular mass of a substance is calculated by taking the sum of the atomic masses of all the atoms in a molecule of respective substance. For example, the molecular mass of water is calculated as −
Atomic mass of hydrogen = 1u
Atomic mass of oxygen = 16 u
The water contains two atoms of hydrogen and one atom of oxygen.
Molecular Mass of Water is = 2 1+ 116 = 18 u (u is the symbol of molecular mass).
Formula Unit Mass
The formula unit mass of a substance is calculated by taking the sum of the atomic masses of all atoms in a formula unit of a compound.
Avogadro Constant or Avogadro Number
Avogadro was an Italian scientist who had given the concept of Avogadro Number (also known as Avogadro Constant).
The number of particles (atoms, molecules, or ions) present in 1 mole of any substance is fixed, and its value always calculated as 6.022 1023.
In 1896, Wilhelm Ostwald had introduced the concept of mole; however, mole unit was accepted to provide a simple way of reporting a large number in 1967.
Law of Conservation of Mass
During a chemical reaction, sum of the masses of the reactants and products remains unchanged, which is known as the Law of Conservation of Mass.
Law of Definite Proportions
In a pure chemical compound, its elements are always present in a definite proportion by mass, which is known as the Law of Definite Proportions.
Chemistry - Structure of the Atom
Introduction
By 1900, it was discovered that the atom was not a simple, indivisible particle, but rather it contains sub-atomic particles.
J.J. Thomson discovered the sub-atomic particle namely electron.

J.J. Thomson was the first person who proposed a model for the structure of an atom.
In 1886, E. Goldstein discovered the presence of new radiations in a gas discharge and named them canal rays.
Another positively charged sub-atomic particle was discovered with experiments of canal rays and named it proton.
Thomsons Model of Atom
Thomson proposed that an atom consists of a positively charged sphere and the electrons (negative charge) are embedded in it (as shown in the image given below).
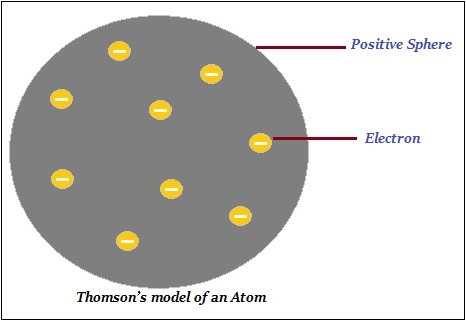
Further, Thomson said that the negative and positive charges are equal in magnitude. Thus, the atom as a whole is electrically neutral.
Rutherfords Model of Atom
E. Rutherford is popular as the Father of nuclear physics.

Rutherford is largely known for his work on radioactivity and the discovery of the nucleus of an atom with the gold foil experiment (as shown in the image given below.
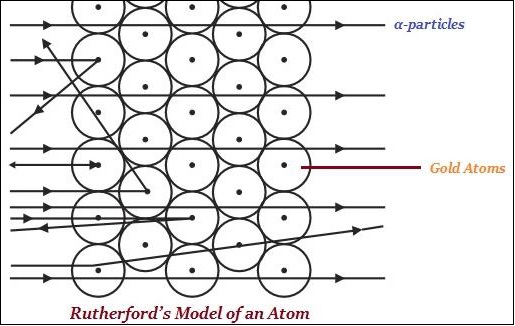
Rutherford said that in an atom, there is a positively charged center known as the nucleus.
Rutherford said that nearly all the mass of an atom exists in in the nucleus.
According to Rutherford, the electrons revolve around the nucleus in well-defined orbits.
Bohrs Model of Atom
Neils Bohr further extended Rutherfords model and improved his drawbacks.

According to Bohr, only certain special orbits known as discrete orbits of electrons, are allowed inside the atom.
Bohr said that electrons do not radiate energy while revolving in discrete orbits.
Bohr named orbits or shells as energy levels (as shown in the image given below).
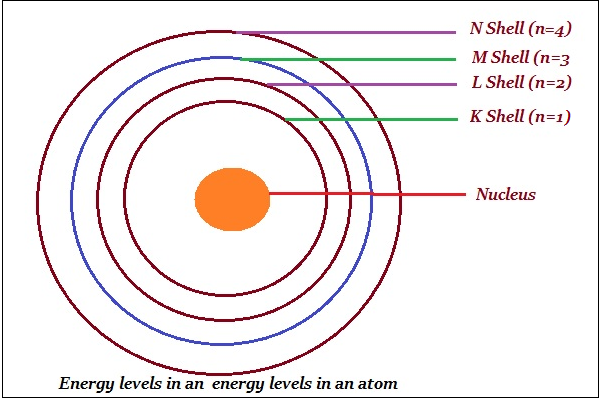
Bohr represented these orbits or shells are by the letters K, L, M, N, or the numbers, n = 1,2,3,4,.
Neutron
In 1932, J. Chadwick discovered a new sub-atomic particle i.e. neutron.
Neutron has no charge and a mass nearly equal to that of a proton.
Neutrons are present in the nucleus of all atoms, except hydrogen.
Electrons Distributed in Different Orbits (Shells)
The maximum number of electrons that can be present in a shell is given by the formula 2n2.
n is the orbit number or energy level index, i.e. 1, 2, 3,.
According to the given formula −
First orbit i.e. K-shell will be = 2 12 = 2
Second orbit i.e. L-shell will be = 2 22 = 8
Third orbit i.e. M-shell will be = 2 32 = 18
Fourth orbit i.e. N-shell will be = 2 42 = 32
Likewise, the maximum number of electrons that can be accommodated in the outermost orbit is 8.
Electrons are not filled in a given shell, unless the inner shells are filled. It means, the shells are filled in a step-wise manner; starting from inner shell to outer shell.
Valence
The electrons, those are present in the outermost shell of an atom, are known as the valence electrons.
According to Bohr-Bury model, the outermost shell of an atom can have a maximum of 8 electrons.
Atomic Number
The total number of protons, present in the nucleus of an atom, is known as atomic number.
The number of protons of an atom determines the atomic number.
Atomic number is denoted by Z.
Protons and neutrons collectively are known as nucleons.
Mass Number
The sum of the total number of protons and neutrons, present in the nucleus of an atom, is known as mass number.
Isotopes
The atoms of the same element, having the same atomic number but different mass numbers, is known as isotopes. E.g. Hydrogen atom has three isotopes namely protium, deuterium, and tritium.
The chemical properties of isotopes of an atom are similar but their physical properties are different.
Isobars
Atoms of different elements with different atomic numbers, which have the same mass number, are known as isobars. E.g. calciums atomic number is 20and argons atomic number is 18; further, the number of electrons in these atoms is different, but the mass number of both these elements is 40.
Chemistry - Chemical Reactions & Equations
Introduction
A process in which one or more chemical substances react with other chemical substance and converted to one or more different substances is known as chemical reaction.
Chemical Equation
A chemical equation is the symbolic demonstration of a chemical reaction; it is represented through symbols and formulae. E.g.
Magnesium + Oxygen = Magnesium Oxide
Mg + O2 = MgO
The substances magnesium and oxygen are known as reactants and the result of reaction, i.e., magnesium oxide is known as product.
Remember, the total mass of the elements present in the products of a chemical reaction has to be equal to the total mass of the elements present in the reactants.
The number of atoms of each element always remains same, before and after the chemical reaction.
Types of Chemical Reaction
Following are the major types of chemical reaction −
Combination Reaction
Decomposition Reaction
Displacement Reaction
Lets discuss each of them in brief −
Combination Reaction
When two or more substances (i.e. elements or compounds) react to form a single product, such reaction is known as combination reaction. E.g.
CaO(s) +H2O(1)→Ca(OH)2(aq)
(Quick lime) (Slaked lime)
As illustrated in the above reaction, calcium oxide and water reacted (or combined) to form a single product, known as calcium hydroxide.
The chemical reaction in which heat is also released along with the formation of product is known as exothermic chemical reactions.
Decomposition Reaction
The reaction, in which a single reactant breaks down into simpler products, is known as a decomposition reaction. E.g.

In the above given reaction, Ferrous sulphate crystals (i.e. FeSO4, 7H2O), when heated, it loses water and the color of the crystals changes. Finally, it decomposes into ferric oxide (Fe2O3), sulphur dioxide (SO2) and sulphur trioxide (SO3).
Displacement Reaction
The reaction, in which an element displaces or removes another element, is known as displacement reaction. E.g.
Fe(s)+ CuSO4(aq)→FeSO4(aq)+Cu(s)
(Copper sulphate)(Iron sulphate)
In the above given reaction, iron displaced copper from copper sulphate solution and forms Iron sulphate.
Oxidation and Reduction
If a substance gains oxygen during a reaction, it is known as oxidation. On the other hand, in a reaction, if a substance loses oxygen, it is known as reduction. E.g.

In the above given reaction, the copper oxide loses oxygen and hence reduced (i.e. reduction); on the other hand, hydrogen gains oxygen and hence oxidized (i.e. oxidation).
Corrosion
When a metal is attacked by substances found in the immediate environment, such as moisture, acids, etc., it is known as corrosion. E.g. the black coating on silver, the green coating on copper, etc.,

Rancidity
When fats and oils are getting oxidized, the process is known as rancidity. Their smell, taste, color, etc. also change; likewise, it made food unsafe for consumption.
Chemistry - Acids, Bases, and Salts
Introduction
We taste food sour and bitter, it is only because of presence of acids and bases respectively.
Litmus Solution
Litmus, which is extracted from lichen, has purple color (see the image given below), but the condition is when it is neither acidic nor basic, i.e. neutral.

Litmus basically is a plant belongs to Thallophyta, and in chemical experiment, it is commonly used as an indicator.
The substances, which odor changes in acidic or basic media, are known as olfactory indicators.
Acid or Base in a Water Solution
The hydrogen ions in HCl are produced because of the presence of water. Secondly, the separation of H+ ion from the HCl molecules cannot be done in the absence of water. The chemical formula is illustrated below
HCl + H2O → H3O+ + Cl
Furthermore, hydrogen ions cannot exist alone, but they can exist in presence of water molecules. Therefore, hydrogen ions are shown as H+(aq) or hydronium ion (H3O+). The chemical formula is −
H+ + H2O → H3O+
The bases which are soluble in water are known as alkalis. But all bases are not soluble in water.
If water is added to a concentrated acid, then the heat is generated.
Mixing an acid or base with the water results into decrease in the concentration of ions (i.e. H3O+/OH) per unit volume and the process is known as dilution.
pH Scale
A scale, used in measuring the hydrogen ion concentration in a solution, is known as pH scale.
The p in pH stands for potenz, it is a German term, which means power.
pH value is taken simply as a number, which indicates the acidic or basic nature of a solution. So, if the concentration of hydronium ion is higher, then the value of pH would be lower.
The value of pH scale ranges between 0 and 14; so, if pH value is measured 0, it means it is very acidic and if it is 14, then it means it very alkaline.
The neutral value of pH scale is 7.
On a pH scale, values less than 7 represent an acidic solution and values greater than 7 represent a basic solution.
Usually, paper impregnated with the common indicator is used for measuring the pH (see the image given below)−
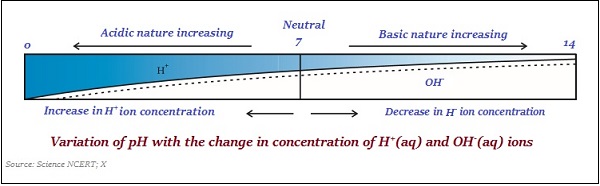
Likewise, the strength of acids and bases substance mainly depends on the number of H+ ions and OH ions produced, respectively.
The following image roughly illustrates (variations in color) the pH value of some of the common substances −
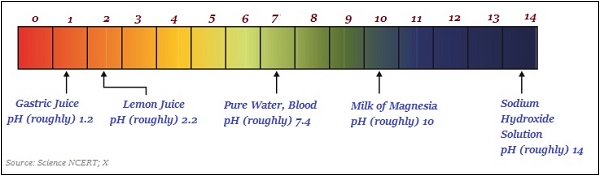
Importance of pH in Everyday Life
The pH value of a human body ranges between 7.0 and 7.8.
The stomach of a human body produces hydrochloric acid that helps in the digestion of food; surprisingly, it does not harm the stomach anyway.
However, when the stomach produces too much acid (known as indigestion), it causes pain and irritation. To get relief from this pain, doctors suggest the use of bases known as antacids.
These antacids neutralize and control the increased amount of acid.
The teeth, which are made up of calcium phosphate, is the hardest substance in the body. However, when the pH in the mouth decreases (below 5.5), it corrodes the teeth.
The salt, normally, is formed by the combination of hydrochloric acid and sodium hydroxide solution; and, the combination is known as sodium chloride.
When the pH value of rain water is measured as less than 5.6, it is known as acid rain.
When acid rain flows into the rivers, then it also lowers the pH of the river water
The acidic river water is threat for the survival of aquatic life.
Bleaching Powder
Bleaching powder is produced by the action of chlorine on dry slaked lime [Ca(OH)2] and it is represented as CaOCl2.
Bleaching powder is normally used in textile industry, paper factory, chemical industry, and disinfecting the drinking water.
Baking Soda
The baking soda is commonly used in the kitchen in order to cook tasty crispy food items. It also cooks some food items faster.
The chemical name of baking soda is sodium hydrogencarbonate and formula is NaHCO3.
Washing Soda
Recrystallization of sodium carbonate results into washing soda.
The chemical formula of washing soda is Na2CO3.10H2O.
Washing soda is commonly used in glass, soap, and paper industries.
Plaster of Paris
Plaster of Paris is a white powder that doctors use as plaster for supporting fractured bones.
The chemical name of plaster of paris is calcium sulphate hemihydrate and chemical formula is 2CaSO4.H2O.
Materials: Metals and Non-Metals 1
Introduction
The metals can be distinguished from the non-metals on the basis of their chemical and physical properties.
The property of metals by which they can be beaten into thin sheets is known as malleability.
The property of metal by which it can be drawn into wires is known as ductility.
The metals are normally hard, malleable, lustrous, ductile, sonorous, and good conductors of heat and electricity. E.g. iron, copper, calcium, aluminum, magnesium, etc.

The materials, which are not sonorous and are poor conductors of heat and electricity, are known as non-metals. E.g. sulphur, carbon, oxygen, phosphorus, etc.
Some metals, such as sodium and potassium are soft and can be cut with a knife.
Mercury is the only metal, which remains in liquid state at room temperature.
When sulphur dioxide is dissolved in water, sulphurous acid is formed. Illustration - Sulphur dioxide (SO2) + Water (H2O) → Sulphurous acid (H2SO3).
Oxides of non-metals are acidic in nature.
The sulphurous acid changes blue litmus paper red.
Phosphorus is a very reactive non-metal and it catches fire whenever exposed to air.
To prevent the contact of phosphorus with atmospheric oxygen, Phosphorus is stored in water.
On burning, metals easily react with oxygen and produce metal oxides, these are basic in nature.
Non-metals react with oxygen and produce non- metallic oxides; these are acidic in nature.
Some metals react with water and produce metal hydroxides and hydrogen gas.
Nonmetals normally do not react with water.
Metals also react with acids and produce hydrogen gas and metal salts.
Non-metals normally do not react with acids.
Uses of Metals and Nonmetals
Metals are used in making machinery, airplanes, automobiles, trains, satellites, industrial gadgets, cooking utensils, water boilers, etc.
Non-metals are used in fertilizers to improve the growth of plants.
Non-metal are used in water purification.
Non-metals are used in crackers.
Chemistry - Metals and Non-Metals 2
Introduction
A solid material, which is typically hard, malleable, shiny, fusible, and ductile, is known as metals. E.g. iron, copper, aluminum, magnesium, sodium, lead, zinc, etc.
Normally, metals have good electrical and thermal conductivity.

Metals, in their pure state, have a shining surface, known as metallic luster.
Metals can be beaten into thin sheets; this property is known as malleability.
The property of metals to be drawn into the thin wires is known as ductility. E.g. gold is the most ductile metal.
Silver and copper are the best heat conductor.
Non-Metals
The non-metals are normally found in either solids or gases states. However, bromine is an exception that found in liquid state.
Some of the major examples of non-metals are carbon, sulphur, iodine, oxygen, hydrogen, etc.

Facts of Metals and Non-metals
All metals exist in the solid form at room temperature, except mercury.
Gallium and caesium have very low melting points; these two metals get melt even on palm.
Iodine is a non-metal, but it is lustrous (lustrous is the property of metal).
Carbon is a non-metal that can exist in different forms. Each form is called an allotrope.
Diamond is an allotrope of carbon and it is the hardest natural substance known.
The melting and boiling point of diamond is very high.
Graphite is also allotrope of carbon; it is a conductor of electricity.
Alkali metals, such as lithium, potassium, sodium, are the examples of soft metals, as they can be cut with a knife.
Nearly all metals when combined with oxygen, it forms metal oxides.
Different metals have different frequency of reaction; some react slow, but some react very fast. E.g. potassium and sodium are very reactive and they catch fire only if kept in the open.
Therefore, potassium and sodium are kept immersed in kerosene oil so that they cannot catch fire.
However, among all metals, sodium (most likely), is the most reactive metal.
Anodizing is a process of forming a thick protective oxide layer of aluminum and it protects from corrosion.
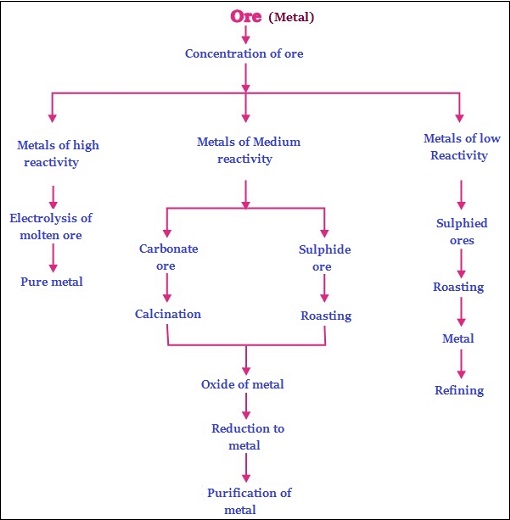
The elements or compounds that occur naturally in the crust (upper layer) of the earth, are known as minerals.
The minerals in raw form is known as ores. E.g. gold, silver, iron etc. (iron ore shown in the image given below) −

The ores, which are extracted from the earth, are usually contaminated with large amounts of impurities such as mix with some elements, soil, sand, etc., known as gangue.
Based on the reactive nature and extraction from the ores, metals can be categorized as −
Chemistry - Carbon and its Compounds
Introduction
Carbon plays very important roles for all living beings.
The amount of carbon in the earths crust is merely 0.02%, which is available in the form of minerals such as carbonates, hydrogen-carbonates, coal, and petroleum.
The presence of carbon in the atmosphere of the earth is 0.03%, in the form of carbon dioxide.
Compounds of Carbon
Almost all carbon compounds (except a few) are poor conductors of the electricity.
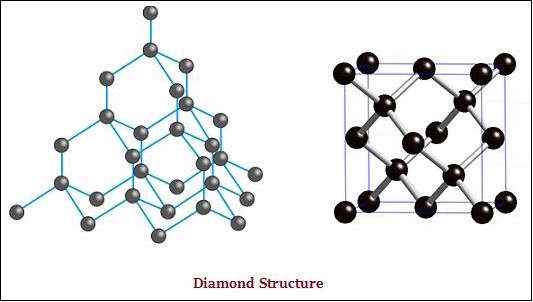
The diamond and graphite both are formed by carbon atoms; however, the difference lies between them in the manner in which the carbon atoms are bonded to one another.
In diamond, each atom of the carbon, is bonded to four other carbon atoms and form a rigid three-dimensional structure (see the image given below).
In graphite, each atom of the carbon, is bonded to three other carbon atoms in the same plane, which gives a hexagonal array (see the image given below) −
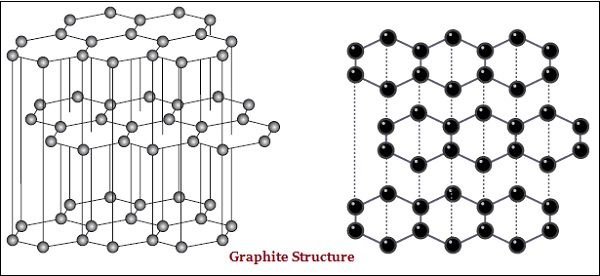
There is also difference in some physical structure of diamond and graphite.
Diamond is the hardest substance known whereas graphite is smooth and slippery substance.
Graphite is good conductor of electricity whereas diamond is not.
Following table illustrates the structures of compounds of carbon and hydrogen −
| Name | Formula | Structure |
|---|---|---|
| Methane | CH4 | 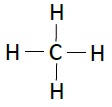 |
| Ethane | C2H6 | 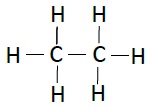 |
| Propane | C3H8 | 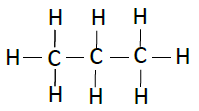 |
| Butane | C4H10 | 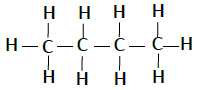 |
| Pentane | C5H12 | 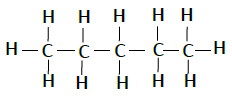 |
| Hexane | C6H14 | 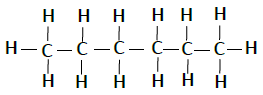 |
The compounds, which has identical molecular formula, but different structures, are known as structural isomers (see the structure Butane given below).
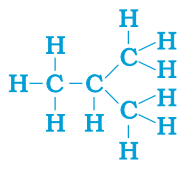
The saturated hydrocarbons are known as alkanes.
The unsaturated hydrocarbons, which comprise of one or more double bonds, are known as alkenes.
The unsaturated hydrocarbons, which comprise of one or more triple bonds, are known as alkynes.
Use of Alcohol as Fuel
Sugarcane plants very efficient convert sunlight into chemical energy and its juice can be used to prepare molasses.
When molasses is fermented, it produces alcohol (ethanol).
Some of the countries now using alcohol as an additive in petrol, as it is a cleaner fuel.
These alcohol, on burning in sufficient air (oxygen), gives rise to only carbon dioxide and water.
Esters
Esters are sweet-smelling substances, which are most commonly formed by reaction of an acid and an alcohol (see the image below illustrating the formation of esters).
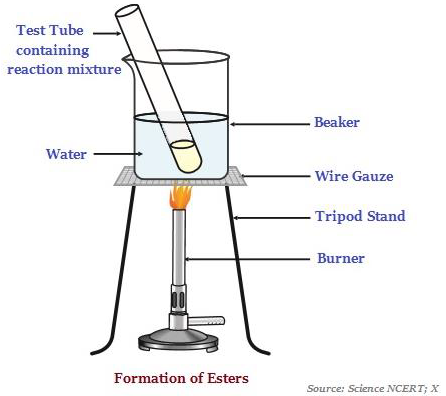
When esters react in the presence of an acid or a base, it gives back the alcohol and carboxylic acid.
The reaction of esters with an acid or a base, is known as saponification because it is used in the preparation of soap.
The molecules of soap normally are sodium or potassium salts of long-chain carboxylic acids.
Interestingly, the ionic-end of soap dissolves in water whereas the carbon chain dissolves in oil. This typical features of the soap molecules forms structures known as micelles (see the image given below)
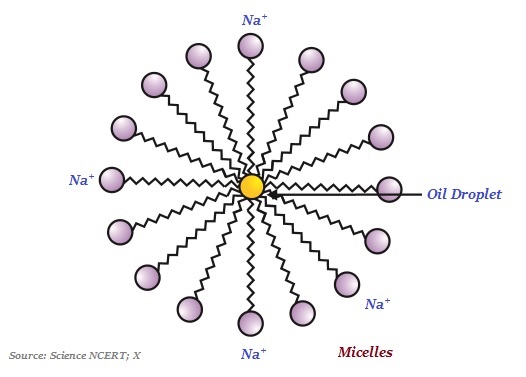
In micelles, one end of the molecules is towards the oil droplet whereas the ionic-end remains outside.
The soap micelle helps in dissolving the dirt in water; likewise, the clothes get cleaned.
On the other hand, detergents are usually ammonium or sulphonate salts of long chain carboxylic acids, which remain effective even in hard water.
Detergents are customarily used to make shampoos and some other products for cleaning clothes.
Periodic Classification Of Elements
Introduction
There are about 115 elements have been known to us till today.
Based on their properties, all the elements are arranged in order, known as periodic table.
Johann Wolfgang Dbereiner, a German scientist, first attempted to arrange the elements in 1817.
John Newlands, an English scientist, also attempted to arrange the then known elements (in 1866).
John Newlands had followed the order of increasing atomic masses to arrange the elements.
Newlands started with the element having the lowest atomic mass (such as hydrogen) and ended at thorium, which was the 56th element (at his time).

Newlands arrangement of elements is known as Law of Octaves, as in his arrangement every eight element had the properties similar to that of the first. E.g. the properties of lithium and sodium were found to be the same.
| Sa (do) | re (re) | Ga (mi) | Ma (fa) | Pa (so) | da (la) | ni (ti) |
|---|---|---|---|---|---|---|
| H | Li | Be | B | C | N | O |
| F | Na | Mg | Al | Si | P | S |
| Cl | K | Ca | Cr | Ti | Mn | Fe |
| Co & Ni | Cu | Zn | Y | In | As | Se |
| Br | Rb | Sr | Ce & La | Zr |
Newlands also compared it with the octaves that found in music (see the table given above).
In the Indian music, the seven musical notes are sa, re, ga, ma, pa, da, ni; however, in the west, the musical notes are do, re, mi, fa, so, la, ti.
Further, in order to fit some elements into his Table, Newlands put two elements in the same cell (see the table given above cobalt & nickel kept in same cell), but this technique did not work, as they have different properties.
However, the law of octave had limitation, as was applicable up to calcium only; and, after calcium every eighth element had not the properties similar to that of the first.
Mendelevs Periodic Table
Dmitri Ivanovich Mendelev, a Russian chemist, who successfully attempted to arrange the elements.
Mendelev arranged the elements based on their (elements) fundamental property, the atomic mass, as well as on the similarity of chemical properties.
During the Mendeleevs time, only 63 elements were known.
Mendelevs Periodic Table consists of vertical columns known as groups and horizontal rows known as periods.

Mendelevs Periodic Law states that
The properties of elements are the periodic function of their atomic masses.
Mendelev arranged the sequence in inverted fashion so that elements with similar properties could be grouped together.
Mendelev left space for some elements, which were not discovered at that time; he boldly predicted about the existence of future elements.
One of the biggest limitation of Mendelevs Periodic formula is - no fixed position has been assigned to hydrogen in the Periodic Table.
Modern Periodic Table
In 1913, Henry Moseley, an English physicist discovered that the atomic number of an element is a more fundamental property in comparison to its atomic mass.
Based on Moseleys discovery, Mendelevs Periodic Law was modified and atomic number was adopted as the basis of Modern Periodic Table.
The Modern Periodic Law states −
Properties of elements are a periodic function of their atomic number.
18 vertical columns known as groups and 7 horizontal rows known as periods are defined in the Modern Periodic Table.
In Modern Periodic Table, the elements are arranged in such a way that it shows periodicity of properties such as atomic size, valency, or combining capacity and metallic and non-metallic characteristics (of elements).
In Modern Periodic Table, the metallic character decreases across a period and increases down the group.
On the other hand, non-metals are electronegative, as they tend to form bonds by gaining electrons.
In Modern Periodic Table, the non-metals are placed on the right-hand side (from the top).
Chemistry - Synthetic Fibres and Plastics
Introduction
The clothes that we wear are made up of fabrics and the fabrics are made from fibers, which is obtained from natural or artificial sources.
The natural source of fibers is cotton, wool, silk, etc., which are obtained from plants or animals.
The synthetic fibers are made by human beings; therefore, these are called synthetic or man-made fibers.
A synthetic fiber is usually a chain of small units those joined together; each small unit is a chemical substance.
Types of Synthetic Fibers
The artificial silk is usually known as Rayon.
Rayon (fiber) was obtained by chemical treatment of wood pulp.
The fiber, prepared from coal, water and air, is known as Nylon.

Nylon was the first fully synthetic fiber.
Polyester is also a synthetic fiber; it is wrinkle free fiber. E.g. Terylene.
PET is one of the familiar form of polyesters and it is used for making utensils, bottles, films, wires, and many other useful products.
Polyester (Poly + ester) is made up of the repeating units of a chemical known as an ester.
Plastic is also a sort of polymer like the synthetic fiber.
Polythene (Poly + ethene) is a common example of a plastic.
There are some plastics, which when molded once, cannot be softened by heating; therefore, these are known as thermosetting plastics. E.g. Bakelite and melamine.
Bakelite is a poor conductor of heat and electricity; therefore, it is used in making electrical switches, handles of various utensils, etc.
Melamine resists fire and can tolerate heat better than other plastics; therefore, it is used for making floor tiles, kitchenware, and fabrics.
A material, which gets decomposed through the natural processes, e.g. action by bacteria, is known as biodegradable.
A material, which cannot be easily decomposed by natural processes, is known as non-biodegradable.
Plastic is not an environment friendly.
Chemistry - Coal and Petroleum
Introduction
The resources, which are present in unlimited quantity in nature and are not likely to be exhausted by human activities, are known as Inexhaustible Natural Resources. E.g. sunlight, air.
The resources, which are present in limited quantity in nature and are likely to be exhausted by human activities, are known as Exhaustible Natural Resources. E.g. forests, wildlife, minerals, coal, petroleum, natural gas etc.
Exhaustible natural resources were formed from the dead remains of living organisms (fossils); therefore, these natural resources are also known as fossil fuels. E.g. coal, petroleum and natural gas.
Coal
Coal is hard as stone and black in color.
Coal is one of the fuels used to cook food.

Coal is used in thermal power plants to produce electricity.
Under high pressure and high temperature, the dead plants those got buried inside the Earth, got slowly converted into coal.
Coal contains mainly carbon.
The slow process of conversion of dead vegetation into coal is known as carbonization.
Coal is formed from the remains of vegetation; therefore, it is also known as fossil fuel.
When coal burns, it produces mainly carbon dioxide gas.
When coal is processed in industry, it produces some useful products such as coke, coal tar, and coal gas.
Coke is a hard, porous, and black substance.
Coke is pure form of carbon.
Coke is largely used in the manufacturing of steel and in the extraction of many metals.
Coal tar is a black, thick liquid with unpleasant smell.
Coal tar is mixture of about 200 substances.
The products, those are obtained from coal tar, are used as starting materials for manufacturing various substances used in everyday life and in industry. E.g. explosives, paints, roofing materials, synthetic dyes, drugs, perfumes, plastics, photographic materials, etc.
Naphthalene balls, obtained from coal tar, are used to repel moths and other insects.
Bitumen, obtained from petroleum product, is used in place of coal-tar for metalling the roads.
During the processing of coal to get coke, coal gas is obtained.
In 1810, for the first time in London, UK, coal gas was used for street lighting and in 1820, in New York, USA.
At present, coal gas is used as a source of heat.
Petroleum
Petrol and diesel are obtained from a natural resource known as petroleum.
Petroleum was formed from the organisms living in the sea.
Over millions of years (the dead organisms buried inside the earth), in the presence high temperature, high pressure, and in the absence of air, the dead organisms transformed into petroleum and natural gas.
In 1859, the worlds first oil well was drilled in Pennsylvania, USA.
In 1867, oil was stuck at Makum in Assam, India.
In India, petroleum is largely found in Assam, Gujarat, Mumbai High, Maharashtra, and in the river basins of Godavari and Krishna.
The following image illustrates the layer of gas and oil −
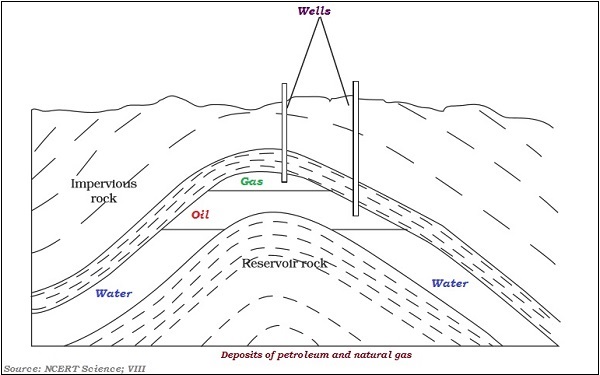
Petroleum is a mixture of various constituents such as petrol, petroleum gas, diesel, lubricating oil, paraffin wax, etc.
The process of separating the various constituents of petroleum is known as refining.
The different useful substances, which are obtained from the petroleum and natural gas, are known as Petrochemicals.
Petrochemicals are used in the manufacturing of detergents, fibers (polyester, nylon, acrylic etc.), polythene and other man-made plastics.
Hydrogen gas, which is obtained from natural gas, is used in the production of fertilizers (urea).
Because of having the great commercial importance, petroleum is also known as black gold.
Natural gas is normally stored under high pressure and hence known as Compressed Natural Gas (CNG).
CNG is used for power generation and fuel for vehicles.
The following table illustrates various constituents of petroleum and their uses −
| Constituents of petroleum | Uses |
|---|---|
| Petroleum Gas in Liquid form (LPG) | Fuel for home and industry |
| Petrol | Motor fuel, aviation fuel, solvent for dry cleaning |
| Diesel | Fuel for heavy motor vehicles, electric generators |
| Kerosene | Fuel for stoves, lamps and for jet aircrafts |
| Lubricating oil | Lubrication |
| Paraffin wax | Ointments, candles, Vaseline, etc. |
| Bitumen | Paints, road surfacing |
Chemistry - Combustion and Flame
Introduction
A chemical process in which a substance reacts with oxygen and give off heat is known as combustion.
The substance that undergoes combustion is called as combustible or fuel.
The fuel can be in the form of solid, liquid, or gas.
During the combustion, light is also given off either in the form of a flame or as a glow.

The substances which vaporize during burning time, give flames.
There are three different zones of a flame dark zone, luminous zone and non-luminous zone.
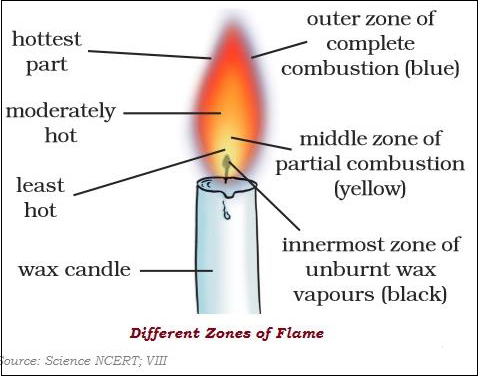
Different substances catch fire at different temperatures.
The lowest temperature at which a substance catches fire is known as its ignition temperature.
A match contains antimony trisulphide and potassium chlorate.
The rubbing surface of match contains powdered glass and a little red phosphorus.
Red phosphorus is much less dangerous.
When the match stick is struck against the rubbing surface, some red phosphorus gets converted into white phosphorus; the process immediately reacts with potassium chlorate present in the matchstick head and produce enough heat to ignite antimony trisulphide; likewise, combustion starts.
The substances, which have very low ignition temperature and can easily catch fire with a flame, are known as inflammable substances. E. g. petrol, alcohol, Liquified Petroleum Gas (LPG), etc.
Fire Extinguisher
Water is the most common fire extinguisher.
Water, as fire extinguisher, works only when things like wood and paper are on fire.
If electrical equipment is on fire, water may conduct electricity and damage those trying to douse the fire.
Water is also not a good extinguisher for fires involving oil and petrol.
For fires that involve electrical equipment and inflammable materials such as petrol, Carbon Dioxide (CO2) is the best extinguisher.

One of the ways to get CO2 is to release plenty of dry powder of chemicals such as sodium bicarbonate (baking soda) or potassium bicarbonate.
Phosphorus burns in air at room temperature.
The amount of heat energy produced on complete combustion of 1 kg of a fuel is known as its calorific value.
The calorific value of a fuel is measured in a unit called kilojoule per kg (kJ/kg).
The following table illustrates the Calorific Values of Different Fuels −
| Fuel | Calorific Value (kJ/kg) |
|---|---|
| Cow dung cake | 6000-8000 |
| Wood | 17000-22000 |
| Coal | 25000-33000 |
| Petrol | 45000 |
| Kerosene | 45000 |
| Diesel | 45000 |
| Methane | 50000 |
| CNG | 50000 |
| LPG | 55000 |
| Biogas | 35000-40000 |
| Hydrogen | 150000 |
Combustion of most fuels releases carbon dioxide in the environment.
Increased concentration of carbon dioxide in the air is most likely causes global warming.
The rise in temperature of the atmosphere of the earth is known as Global Warming.
Global warming causes melting of polar glaciers, which leads to a rise in the sea level that ultimately causing floods in the coastal regions.
Oxides of sulphur and nitrogen dissolve in rain water and form acids; such type of rain is known as acid rain.