- Chemistry - Home
- Matter In Our Surroundings
- Is Matter Around Us Pure
- Chemistry - Atoms & Molecules
- Chemistry - Structure Of The Atom
- Chemical Reactions and Equations
- Chemistry - Acids, Bases, and Salts
- Materials: Metals and Non-Metals I
- Chemistry - Metals & Non-Metals II
- Carbon & its Compounds
- Periodic Classification Of Elements
- Synthetic Fibres and Plastics
- Chemistry - Coal And Petroleum
- Chemistry - Combustion And Flame
Chemistry - Metals and Non-Metals 2
Introduction
A solid material, which is typically hard, malleable, shiny, fusible, and ductile, is known as metals. E.g. iron, copper, aluminum, magnesium, sodium, lead, zinc, etc.
Normally, metals have good electrical and thermal conductivity.

Metals, in their pure state, have a shining surface, known as metallic luster.
Metals can be beaten into thin sheets; this property is known as malleability.
The property of metals to be drawn into the thin wires is known as ductility. E.g. gold is the most ductile metal.
Silver and copper are the best heat conductor.
Non-Metals
The non-metals are normally found in either solids or gases states. However, bromine is an exception that found in liquid state.
Some of the major examples of non-metals are carbon, sulphur, iodine, oxygen, hydrogen, etc.

Facts of Metals and Non-metals
All metals exist in the solid form at room temperature, except mercury.
Gallium and caesium have very low melting points; these two metals get melt even on palm.
Iodine is a non-metal, but it is lustrous (lustrous is the property of metal).
Carbon is a non-metal that can exist in different forms. Each form is called an allotrope.
Diamond is an allotrope of carbon and it is the hardest natural substance known.
The melting and boiling point of diamond is very high.
Graphite is also allotrope of carbon; it is a conductor of electricity.
Alkali metals, such as lithium, potassium, sodium, are the examples of soft metals, as they can be cut with a knife.
Nearly all metals when combined with oxygen, it forms metal oxides.
Different metals have different frequency of reaction; some react slow, but some react very fast. E.g. potassium and sodium are very reactive and they catch fire only if kept in the open.
Therefore, potassium and sodium are kept immersed in kerosene oil so that they cannot catch fire.
However, among all metals, sodium (most likely), is the most reactive metal.
Anodizing is a process of forming a thick protective oxide layer of aluminum and it protects from corrosion.
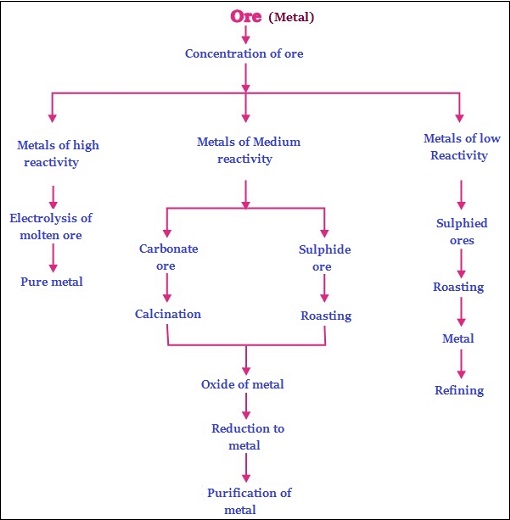
The elements or compounds that occur naturally in the crust (upper layer) of the earth, are known as minerals.
The minerals in raw form is known as ores. E.g. gold, silver, iron etc. (iron ore shown in the image given below) −

The ores, which are extracted from the earth, are usually contaminated with large amounts of impurities such as mix with some elements, soil, sand, etc., known as gangue.
Based on the reactive nature and extraction from the ores, metals can be categorized as −