
- Chemistry - Home
- Matter In Our Surroundings
- Is Matter Around Us Pure
- Chemistry - Atoms & Molecules
- Chemistry - Structure Of The Atom
- Chemical Reactions and Equations
- Chemistry - Acids, Bases, and Salts
- Materials: Metals and Non-Metals I
- Chemistry - Metals & Non-Metals II
- Carbon & its Compounds
- Periodic Classification Of Elements
- Synthetic Fibres and Plastics
- Chemistry - Coal And Petroleum
- Chemistry - Combustion And Flame
Chemistry - Carbon and its Compounds
Introduction
Carbon plays very important roles for all living beings.
The amount of carbon in the earths crust is merely 0.02%, which is available in the form of minerals such as carbonates, hydrogen-carbonates, coal, and petroleum.
The presence of carbon in the atmosphere of the earth is 0.03%, in the form of carbon dioxide.
Compounds of Carbon
Almost all carbon compounds (except a few) are poor conductors of the electricity.
The diamond and graphite both are formed by carbon atoms; however, the difference lies between them in the manner in which the carbon atoms are bonded to one another.
In diamond, each atom of the carbon, is bonded to four other carbon atoms and form a rigid three-dimensional structure (see the image given below).
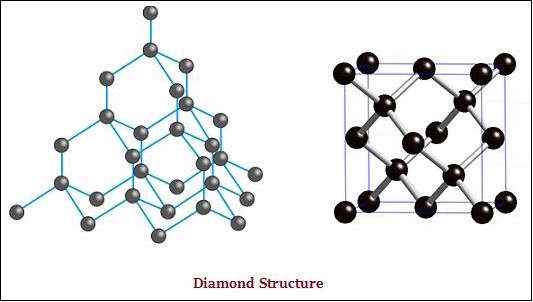
In graphite, each atom of the carbon, is bonded to three other carbon atoms in the same plane, which gives a hexagonal array (see the image given below) −
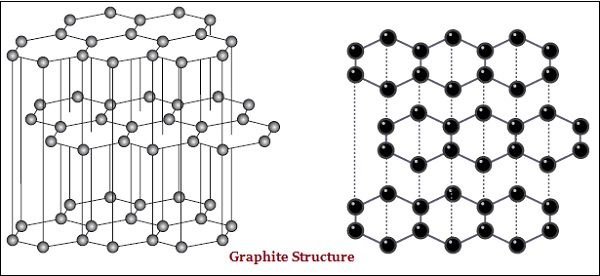
There is also difference in some physical structure of diamond and graphite.
Diamond is the hardest substance known whereas graphite is smooth and slippery substance.
Graphite is good conductor of electricity whereas diamond is not.
Following table illustrates the structures of compounds of carbon and hydrogen −
| Name | Formula | Structure |
|---|---|---|
| Methane | CH4 | 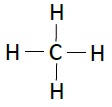 |
| Ethane | C2H6 | 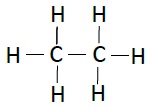 |
| Propane | C3H8 | 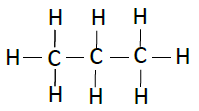 |
| Butane | C4H10 | 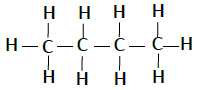 |
| Pentane | C5H12 | 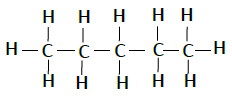 |
| Hexane | C6H14 | 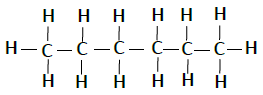 |
The compounds, which has identical molecular formula, but different structures, are known as structural isomers (see the structure Butane given below).
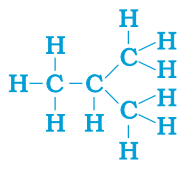
The saturated hydrocarbons are known as alkanes.
The unsaturated hydrocarbons, which comprise of one or more double bonds, are known as alkenes.
The unsaturated hydrocarbons, which comprise of one or more triple bonds, are known as alkynes.
Use of Alcohol as Fuel
Sugarcane plants very efficient convert sunlight into chemical energy and its juice can be used to prepare molasses.
When molasses is fermented, it produces alcohol (ethanol).
Some of the countries now using alcohol as an additive in petrol, as it is a cleaner fuel.
These alcohol, on burning in sufficient air (oxygen), gives rise to only carbon dioxide and water.
Esters
Esters are sweet-smelling substances, which are most commonly formed by reaction of an acid and an alcohol (see the image below illustrating the formation of esters).
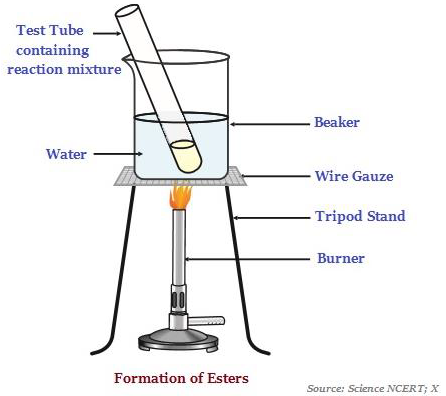
When esters react in the presence of an acid or a base, it gives back the alcohol and carboxylic acid.
The reaction of esters with an acid or a base, is known as saponification because it is used in the preparation of soap.
The molecules of soap normally are sodium or potassium salts of long-chain carboxylic acids.
Interestingly, the ionic-end of soap dissolves in water whereas the carbon chain dissolves in oil. This typical features of the soap molecules forms structures known as micelles (see the image given below)
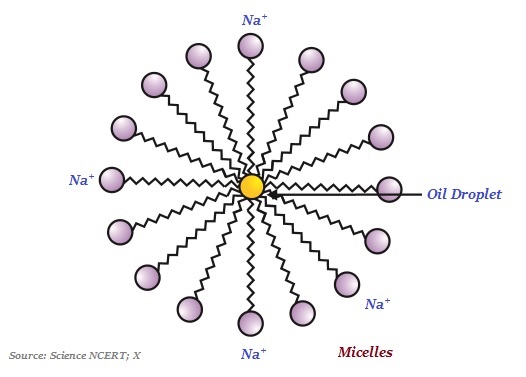
In micelles, one end of the molecules is towards the oil droplet whereas the ionic-end remains outside.
The soap micelle helps in dissolving the dirt in water; likewise, the clothes get cleaned.
On the other hand, detergents are usually ammonium or sulphonate salts of long chain carboxylic acids, which remain effective even in hard water.
Detergents are customarily used to make shampoos and some other products for cleaning clothes.