
- Chemistry - Home
- Matter In Our Surroundings
- Is Matter Around Us Pure
- Chemistry - Atoms & Molecules
- Chemistry - Structure Of The Atom
- Chemical Reactions and Equations
- Chemistry - Acids, Bases, and Salts
- Materials: Metals and Non-Metals I
- Chemistry - Metals & Non-Metals II
- Carbon & its Compounds
- Periodic Classification Of Elements
- Synthetic Fibres and Plastics
- Chemistry - Coal And Petroleum
- Chemistry - Combustion And Flame
Chemistry - Acids, Bases, and Salts
Introduction
We taste food sour and bitter, it is only because of presence of acids and bases respectively.
Litmus Solution
Litmus, which is extracted from lichen, has purple color (see the image given below), but the condition is when it is neither acidic nor basic, i.e. neutral.

Litmus basically is a plant belongs to Thallophyta, and in chemical experiment, it is commonly used as an indicator.
The substances, which odor changes in acidic or basic media, are known as olfactory indicators.
Acid or Base in a Water Solution
-
The hydrogen ions in HCl are produced because of the presence of water. Secondly, the separation of H+ ion from the HCl molecules cannot be done in the absence of water. The chemical formula is illustrated below
HCl + H2O → H3O+ + Cl
-
Furthermore, hydrogen ions cannot exist alone, but they can exist in presence of water molecules. Therefore, hydrogen ions are shown as H+(aq) or hydronium ion (H3O+). The chemical formula is −
H+ + H2O → H3O+
The bases which are soluble in water are known as alkalis. But all bases are not soluble in water.
If water is added to a concentrated acid, then the heat is generated.
Mixing an acid or base with the water results into decrease in the concentration of ions (i.e. H3O+/OH) per unit volume and the process is known as dilution.
pH Scale
A scale, used in measuring the hydrogen ion concentration in a solution, is known as pH scale.
The p in pH stands for potenz, it is a German term, which means power.
pH value is taken simply as a number, which indicates the acidic or basic nature of a solution. So, if the concentration of hydronium ion is higher, then the value of pH would be lower.
The value of pH scale ranges between 0 and 14; so, if pH value is measured 0, it means it is very acidic and if it is 14, then it means it very alkaline.
The neutral value of pH scale is 7.
On a pH scale, values less than 7 represent an acidic solution and values greater than 7 represent a basic solution.
Usually, paper impregnated with the common indicator is used for measuring the pH (see the image given below)−
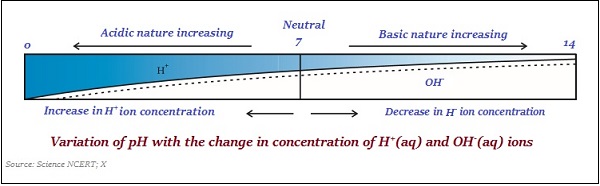
Likewise, the strength of acids and bases substance mainly depends on the number of H+ ions and OH ions produced, respectively.
The following image roughly illustrates (variations in color) the pH value of some of the common substances −
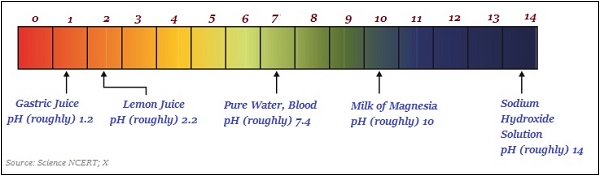
Importance of pH in Everyday Life
The pH value of a human body ranges between 7.0 and 7.8.
The stomach of a human body produces hydrochloric acid that helps in the digestion of food; surprisingly, it does not harm the stomach anyway.
However, when the stomach produces too much acid (known as indigestion), it causes pain and irritation. To get relief from this pain, doctors suggest the use of bases known as antacids.
These antacids neutralize and control the increased amount of acid.
The teeth, which are made up of calcium phosphate, is the hardest substance in the body. However, when the pH in the mouth decreases (below 5.5), it corrodes the teeth.
The salt, normally, is formed by the combination of hydrochloric acid and sodium hydroxide solution; and, the combination is known as sodium chloride.
When the pH value of rain water is measured as less than 5.6, it is known as acid rain.
When acid rain flows into the rivers, then it also lowers the pH of the river water
The acidic river water is threat for the survival of aquatic life.
Bleaching Powder
Bleaching powder is produced by the action of chlorine on dry slaked lime [Ca(OH)2] and it is represented as CaOCl2.
Bleaching powder is normally used in textile industry, paper factory, chemical industry, and disinfecting the drinking water.
Baking Soda
The baking soda is commonly used in the kitchen in order to cook tasty crispy food items. It also cooks some food items faster.
The chemical name of baking soda is sodium hydrogencarbonate and formula is NaHCO3.
Washing Soda
Recrystallization of sodium carbonate results into washing soda.
The chemical formula of washing soda is Na2CO3.10H2O.
Washing soda is commonly used in glass, soap, and paper industries.
Plaster of Paris
Plaster of Paris is a white powder that doctors use as plaster for supporting fractured bones.
The chemical name of plaster of paris is calcium sulphate hemihydrate and chemical formula is 2CaSO4.H2O.