
- Chemistry - Home
- Matter In Our Surroundings
- Is Matter Around Us Pure
- Chemistry - Atoms & Molecules
- Chemistry - Structure Of The Atom
- Chemical Reactions and Equations
- Chemistry - Acids, Bases, and Salts
- Materials: Metals and Non-Metals I
- Chemistry - Metals & Non-Metals II
- Carbon & its Compounds
- Periodic Classification Of Elements
- Synthetic Fibres and Plastics
- Chemistry - Coal And Petroleum
- Chemistry - Combustion And Flame
Chemistry - Atoms & Molecules
Introduction
Around 500 BC, an Indian Philosopher Maharishi Kanad, first time postulated the concept of indivisible part of matter and named it pramanu.
In 1808, John Dalton used the term atom and postulated the atomic theory to the study of matter.

Daltons Atomic Theory
According to Daltons atomic theory, all matter, whether an element, a compound or a mixture is composed of small particles called atoms.
According to Daltons atomic theory, all matters, whether they are elements, compounds, or mixtures, are composed of small particles known as atoms.
Salient features of Daltons Atomic Theory
All matter is made of very miniscule particles known as atoms.
Atom is an indivisible particle, which cannot be created or destroyed through chemical reaction.
All atoms of an element are identical in mass and chemical properties whereas, atoms of different elements have different masses and chemical properties.
To form a compound, atoms are combined in the ratio of small whole numbers.
In a given compound, the relative number and kinds of atoms are constant.
Atomic Mass
The mass of an atom of a chemical element; it is expressed in atomic mass units (symbol is u).
The atomic mass is roughly equivalent to the number of protons and neutrons present in the atom.
One atomic mass unit is a mass unit equal to the exactly one-twelfth (1/12th) the mass of one atom of carbon-12 and the relative atomic masses of all elements have been calculated with respect to an atom of carbon-12.
Molecule
The smallest particle of an element or a compound, which is capable to exist independently and shows all the properties of the respective substance.
A molecule, normally, is a group of two or more atoms which are chemically bonded together.
Atoms of the same element or of different elements can join (with chemical bond) together to form molecules.
The number of atoms that constitute a molecule is known as its atomicity.
Ion
A charged particle is known as ion; it could be either negative charge or positive charge.
The positively charged ion is known as a cation.
The negatively charged ion is known as an anion.
Chemical Formulae
A chemical formula of a compound demonstrations its constituent elements and the number of atoms of each combining element.
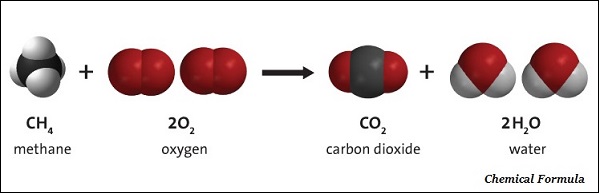
The chemical formula of a compound is the symbolic representation of its Composition.
The combining capacity of an element is known as its valency.
Molecular Mass
The molecular mass of a substance is calculated by taking the sum of the atomic masses of all the atoms in a molecule of respective substance. For example, the molecular mass of water is calculated as −
Atomic mass of hydrogen = 1u
Atomic mass of oxygen = 16 u
The water contains two atoms of hydrogen and one atom of oxygen.
Molecular Mass of Water is = 2 1+ 116 = 18 u (u is the symbol of molecular mass).
Formula Unit Mass
The formula unit mass of a substance is calculated by taking the sum of the atomic masses of all atoms in a formula unit of a compound.
Avogadro Constant or Avogadro Number
Avogadro was an Italian scientist who had given the concept of Avogadro Number (also known as Avogadro Constant).
The number of particles (atoms, molecules, or ions) present in 1 mole of any substance is fixed, and its value always calculated as 6.022 1023.
In 1896, Wilhelm Ostwald had introduced the concept of mole; however, mole unit was accepted to provide a simple way of reporting a large number in 1967.
Law of Conservation of Mass
During a chemical reaction, sum of the masses of the reactants and products remains unchanged, which is known as the Law of Conservation of Mass.
Law of Definite Proportions
In a pure chemical compound, its elements are always present in a definite proportion by mass, which is known as the Law of Definite Proportions.