
 Data Structure
Data Structure Networking
Networking RDBMS
RDBMS Operating System
Operating System Java
Java MS Excel
MS Excel iOS
iOS HTML
HTML CSS
CSS Android
Android Python
Python C Programming
C Programming C++
C++ C#
C# MongoDB
MongoDB MySQL
MySQL Javascript
Javascript PHP
PHP
- Selected Reading
- UPSC IAS Exams Notes
- Developer's Best Practices
- Questions and Answers
- Effective Resume Writing
- HR Interview Questions
- Computer Glossary
- Who is Who
What is Electrolysis? – Definition, Principle, and Process
What is Electrolysis?
The process of electrolysis can be defined in many ways −
A technique that uses a direct electric current to stimulate a non-spontaneous chemical reaction is known as electrolysis.
In other words, the process in which ionic substances are decomposed into simple substances by passing an electric current through them is known as electrolysis.
Or, electrolysis is the process based on the fact that electrical energy can produce chemical changes, i.e., it can cause a chemical reaction to happen.
Principle of Electrolysis
The principle of electrolysis is based on the term "Electrolyte". An electrolyte is a substance containing free ions that make the substance electrically conductive. In general, an electrolyte is an ionic solution, but molten electrolytes and solid electrolytes are also available.
In nature, the atoms of electrolytes are closely bound together but this bond becomes weaker when dissolved and the molecules of electrolyte split into two types of ions viz. cations (positive ions) and anions (negative ions) moving freely in the solution.
Now, if two electrodes are dipped into the electrolyte and connected to the DC power supply. Then, the cations moving freely in the solution are attracted by the cathode and the anions moving freely in the solution are attracted by the anode.
Process of Electrolysis
In order to understand the process of electrolysis, we consider the following example.
Consider a crucible that is filled with water in which two electrodes viz. anode and cathode are immersed and these electrodes are supplied from a source of DC power. The arrangement for the process of electrolysis is shown in the figure.
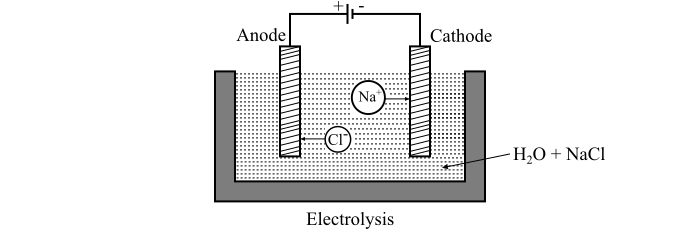
Now, when sodium chloride (NaCl) salt is dissolved in water, it decomposes into positively charged Na+ ions and negatively charged Cl− ions, moving freely in the solution. As the electrodes are connected to the DC supply, thus the positively charged Na+ ions travel towards the cathode and the negatively charged Cl− ions travel towards the anode.
On reaching the cathode, each positively charged sodium (Na+) ion takes one electron from it and forms the sodium metal. Similarly, each of negatively charged chloride ion will give one electrode to the anode and ceases to be anion. Now, the sodium metal deposited at the cathode reacts with water and giving out oxygen and hydrogen chloride, i.e.,
$$\mathrm{4Cl\: +\: 2H_{2}O\to 4HCL\: +\: O_{2}} $$
This process of deposition of sodium at the cathode from the sodium chloride (NaCl) in water is known as electrolysis.
Now, if the cathode is made of sodium, then the hydrogen chloride again reacts with the sodium metal forming sodium chloride and liberating hydrogen gas. This process can also be represented by the following chemical reaction.
$$\mathrm{2HCl\: +\: 2Na\to 2NaCl+H_{2}} $$
Applications of Electrolysis
The electrolysis is widely used for −
Refining of metals like gold, silver, copper, etc.
Extraction of pure metals from their ores like aluminum, copper, zinc, etc.
Various electro-deposition processes like electro-plating, electro-typing, etc.
Manufacturing of various chemicals.

