Article Categories
- All Categories
-
 Data Structure
Data Structure
-
 Networking
Networking
-
 RDBMS
RDBMS
-
 Operating System
Operating System
-
 Java
Java
-
 MS Excel
MS Excel
-
 iOS
iOS
-
 HTML
HTML
-
 CSS
CSS
-
 Android
Android
-
 Python
Python
-
 C Programming
C Programming
-
 C++
C++
-
 C#
C#
-
 MongoDB
MongoDB
-
 MySQL
MySQL
-
 Javascript
Javascript
-
 PHP
PHP
What is Electro-Extraction? – Definition, Process and Applications
What is Electro-Extraction?
Electro-extraction, also known as electro-winning can be defined in several ways as −
Electro-extraction is an electrochemical process that is used for production of metals with commercially acceptable purity.
Electro-extraction is the electro-deposition of metals from their ores that have been in electrolytic solution.
Electro-extraction is the electrolytic process by which metals can be extracted or separated from their ores.
Based upon the physical status of the metal ore, the metal can be extracted in following two ways −
When the ore is in solid state - The ore is treated with strong acid to obtain its salt, again this salt is to be electrolyzed to liberate the pure metal.
When the ore is in liquid state or molten state - The ore is directly electrolyzed in a furnace to liberate the metal.
Electro-Extraction Process
In order to understand the process of electro-extraction, we assume the electro-extraction of aluminum from its ore. The schematic arrangement for the electro-extraction of aluminium is shown in the figure.
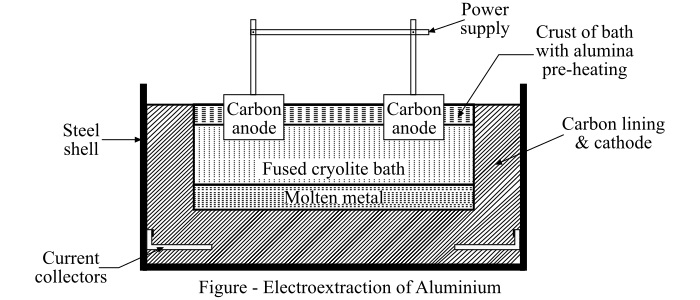
The electro-extraction of aluminium is an example of fused electrolytic process. Aluminium is produced from bauxite containing aluminium oxide or alumina, silica and iron oxide.
The bauxite is first reduced to aluminium oxide by chemical treatment and then it is dissolved in fused cryolite. The cryolite is a solution of aluminium fluoride and fluoride of either sodium, potassium or calcium. The obtained mixture is then electrolyzed. The fusion and electrolysis are accomplished in a large rectangular steel bath lined with carbon. The anodes made of carbon are projected downwards into the bath and the bottom of the bath forms the cathode.
Now, the charge is melted by striking the arc between the carbon anodes and the cathode and then it is maintained in the molten state by the heating action of the electric current flowing through the charge. The liquid metal deposits at the cathode and settle at the bottom of the bath.
For the continuous process, fresh alumina is fed into the bath at short intervals to replace that which has been decomposed by the electric current. The aluminium obtained by this process is 99.5% pure.
Applications of Electro-Extraction
The applications of electro-extraction or electro-winning are listed below −
Electro-extraction is widely used process for extraction of the most common metals like aluminium, copper, zinc, gold, silver, lead, chromium, cobalt, manganese, etc.
Electro-extraction process is used in industries to produce active metals which react strongly with water.
Electro-extraction is also used for separation of heavy metals such as plutonium, cesium, strontium, etc.
Electro-extraction is used for separation of the rare-earth metals and alkali metals.
Electro-extraction is also employed for removing toxic metals from industrial waste streams.


