
 Data Structure
Data Structure Networking
Networking RDBMS
RDBMS Operating System
Operating System Java
Java MS Excel
MS Excel iOS
iOS HTML
HTML CSS
CSS Android
Android Python
Python C Programming
C Programming C++
C++ C#
C# MongoDB
MongoDB MySQL
MySQL Javascript
Javascript PHP
PHP
- Selected Reading
- UPSC IAS Exams Notes
- Developer's Best Practices
- Questions and Answers
- Effective Resume Writing
- HR Interview Questions
- Computer Glossary
- Who is Who
Decomposition Reaction
Introduction
In a decomposition reaction, molecules or compounds break to give two or more simpler new substances.
There are various types of reactions or chemical reactions: combination type, decomposition type, displacement type, double displacement type, combustion reaction, neutralization reaction, precipitation reaction, and redox reaction. In the precipitation or double replacement reaction, an insoluble precipitate(leftover) is formed or settled down the container. This reaction occurs when two or more soluble salts react or combine to give an insoluble precipitate.
The neutralization reaction is a type of reaction where acid (?+), and base (???) react or combine to give salt and water.
We can understand from the example, that hydrochloric acid (???) reacts with sodium hydroxide (????) to give sodium chloride (????) and water. Redox reaction is a combination of oxidation and reduction reactions. A Combustion reaction is nothing but a type of exothermic reaction that usually releases energy in the form of heat. A Synthesis or combination reaction is a reaction where two (2) or more than 2 reactants or elements react or combine to give a single product.
Types of Chemical Reactions
There are various types or different types of chemical reactions. Here, we are mentioning a few of them. Types of the reactions are-
Combination or synthesis type
Decomposition type
Displacement (single) type
Double displacement type
Neutralization type
Combustion type
Precipitation or double replacement reaction
Redox reaction (oxidation-reduction reaction)
Precipitation or Double Replacement Reaction
A precipitation reaction is another type of chemical reaction that usually occurs or takes place in an aqueous (in the presence of water) solution were generally two bonds (mostly ionic) combine to give an insoluble salt or precipitate. This precipitation reaction is an example of a double displacement reaction where a solid residue called precipitate is left behind. One example of such a reaction is the type of reaction that takes place between potassium chloride (???) and silver nitrate (????3), where a compound called silver chloride (????) is precipitated out from the solution. The Reaction is depicted below-
$$\mathrm{AgNO_{3}\:+\:KCl\:\rightarrow\:AgCl\:+\:KNO_{3}}$$
Acid-Base or Neutralization Reaction
A Neutralization reaction is a reaction or a type of chemical reaction where an acid (?+) and a base (???) combine quantitatively to give salt and water. In this reaction, the acidic effect of acid is neutralized by the base and vice versa. During the formation of products, there is the generation of heat. In this (neutralization) reaction, the acidic proton from the acid reacts with the hydroxyl group of the base to give a water molecule. One example of such a reaction is depicted below-
$$\mathrm{NaOH\:+\:HCl\:\rightarrow\:NaCl(salt)\:+\:H_{2}O}$$
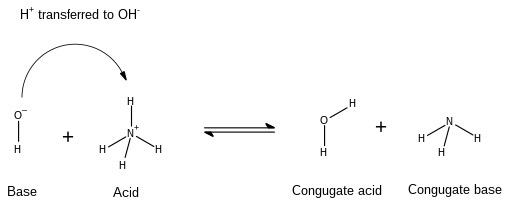
Michael D. Turnbull, Conjugate base reaction, CC BY-SA 4.0
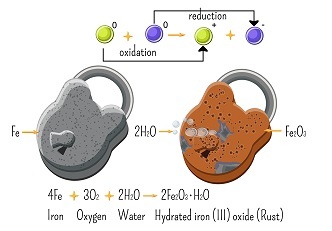
Oxidation-Reduction or Redox Reactions
The reaction in which both the oxidation and reduction process occur at the same time is termed a redox reaction. Here we can define oxidation as the addition of oxygen (?2) or removal (elimination) of hydrogen (?) and, the reduction can be defined as the addition of hydrogen (?) or removal (elimination) of oxygen (?2) atoms. The Reaction between the copper oxide and hydrogen atom is an example of a redox reaction. Rusting of iron is one of the very common and well-known examples of redox reactions.
The Chemical equation for the rusting of iron is depicted below-
$$\mathrm{4Fe\:+\:3O_{2}\:\rightarrow\:2Fe_{2}O_{2}}$$
Combustion Reaction
A Combustion reaction is nothing but an exothermic reaction that releases some amount of energy (chemical) in the form of heat most of the time. These types of reactions generally occur between fuel and an oxidant (or atmospheric oxygen) that produces or gives water, smoke, and energy (chemical) in the form of heat (energy). One example of such a reaction is the burning of methane (??4) to give carbon dioxide (??2) and water (?2?). The reaction for the same has been shown below-
$$\mathrm{CH_{4}\:+\:2O_{2}\:\rightarrow\:CO_{2}\:2H_{2}O}$$
Synthesis Reaction
Synthesis reaction, also called combination (addition) reaction is a type of chemical reaction where two (2) or more reactants or substances are combined to form a different new compound. One example of such a compound is the burning of magnesium in the form of magnesium ribbon to produce magnesium oxide. The reaction for the same is depicted below-
$$\mathrm{Mg\:+\:O_{2}\:\rightarrow\:MgO}$$
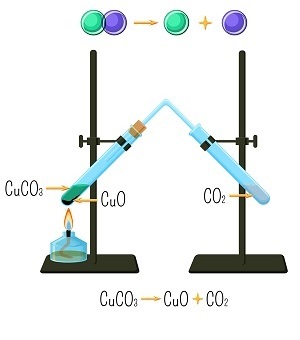
Decomposition Reaction
A Decomposition reaction (opposite of a combination reaction) where a molecule or the compounds break down to give two or simpler elements or chemically new substances. Electrolysis of water is one such example. In the electrolysis of water, water molecules break down to give hydrogen and oxygen. Reaction to the same is as follows-
$$\mathrm{2H_{2}O\:\rightarrow\:2H_{2}\:+\:O_{2}}$$
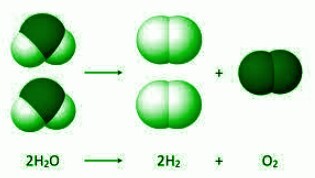
JSquish, Electrolysis of Water, CC BY-SA 3.0
Classification of Decomposition Reaction
Broadly, decomposition reaction can be classified into three major types and those types of reactions are-
Thermal decomposition reaction This is a type of chemical reaction that requires thermal energy for initiating the reaction. So, it is an endothermic reaction as it requires energy. An example of this reaction is-
Electrolytic decomposition reaction This is another type of decomposition reaction (opposite of combination reaction) where energy (chemical) in the form (way) of electricity is required to activate or initiate the reaction. Electrolysis of water is an example of this type of reaction.
Photolytic or photodecomposition reaction This is another type of decomposition reaction (opposite of combination reaction) where energy (form) in the form of light (photons) is required to activate or initiate the reaction. Decomposition of the ozone layer into dioxygen (?2) and elemental oxygen is an example of this type of reaction.
$$\mathrm{CaCO_{3}\:\rightarrow\:CO_{2}\:+\:CaO}$$
$$\mathrm{2H_{2}O\:\rightarrow\:2H_{2}\:+\:O_{2}}$$
$$\mathrm{O_{2}\:+\:hv\:\rightarrow\:O_{2}\:+\:O}$$
Examples of Decomposition Reactions:
Some examples of the decomposition (opposite of combination) reaction are as follows-
Decomposition of an acid called carbonic acid-
Decomposition of calcium carbonate-
Electrolysis of water-
Decomposition of ozone$\mathrm{(O_{3})}$-
$$\mathrm{H_{2}CO_{3}\:\rightarrow\:H_{2}O\:+\:CO_{2}}$$
$$\mathrm{CaCO_{3}\:\rightarrow\:CO_{2}\:+\:CaO}$$
$$\mathrm{2H_{2}O\:\rightarrow\:2H_{2}\:+\:O_{2}}$$
$$\mathrm{O_{3}\:+\:hv\:\rightarrow\:O_{2}\:+\:O}$$
Uses of Decomposition Reaction
Some uses of decomposition reaction are-
It helps in the manufacturing of cement or calcium oxide.
It is also used in the formation of antacids to provide relief from indigestion.
It is also used for the extraction of metals from their ores in the form of oxides, chlorides, etc.
It is used in the welding process called thermite welding.
Importance of Decomposition Reaction
A Decomposition reaction is very important for the digestion of food in the human body because it helps to decompose the carbohydrates and proteins consumed by us into simple sugars in the form of glucose and amino acids, which on further decomposition provides energy in our body.
Conclusion
There are various kinds of reactions or chemical reactions that include combination (addition) reaction, decomposition reaction, precipitation reaction, displacement type reaction, double displacement type reaction, combustion reaction, and redox reaction. A precipitation reaction is a reaction or chemical type reaction that occurs or takes place in an aqueous (in the presence of water) solution where two bonds (mostly ionic) combine to give an insoluble salt or precipitate. A Neutralization reaction is another type of chemical reaction where an acid (?+) and a base (???) react quantitatively with each other to give salt and water. The reaction in which both the oxidation and reduction process occurs simultaneously is termed a redox reaction.
A Combustion (burning) reaction is a type of exothermic reaction that releases some amount of energy in the form of heat most of the time. Synthesis reaction also is a type of chemical reaction where two (2) or more reactants or substances are combined to form a different new compound. A Decomposition type reaction (opposite of the combination reaction) where a molecule or the compounds break down to give two or simpler elements or chemically new substances.
FAQs
1. What do you mean by decomposition reaction?
A Decomposition reaction (combination reaction) is where a molecule or compound breaks down to give two or simpler elements or chemically new substances.
2. What is a Double replacement reaction?
A double replacement reaction also called a precipitation reaction is defined as a chemical reaction or reaction that occurs or takes place in an aqueous (in the presence of water) solution where two bonds (mostly ionic) combine to give an insoluble salt or precipitate.
3. What are the types of decomposition reactions?
Types of decomposition reactions are as follows-
Thermal type decomposition
Electrolytic type decomposition
Photolytic decomposition type
4. Give an example of a Photolytic decomposition reaction?
Decomposition of the ozone layer into dioxygen $\mathrm{(O_{2})}$ and elemental oxygen is an example of a photolytic decomposition reaction.
$$\mathrm{O_{3}\:+\:hv\:\rightarrow\:O_{2}\:+\:O}$$
5. Mention two uses of decomposition reaction?
Two uses of decomposition reaction are-
This is used in the formation of cement or calcium oxide.
This is also used for welding purposes.

