
 Data Structure
Data Structure Networking
Networking RDBMS
RDBMS Operating System
Operating System Java
Java MS Excel
MS Excel iOS
iOS HTML
HTML CSS
CSS Android
Android Python
Python C Programming
C Programming C++
C++ C#
C# MongoDB
MongoDB MySQL
MySQL Javascript
Javascript PHP
PHP
- Selected Reading
- UPSC IAS Exams Notes
- Developer's Best Practices
- Questions and Answers
- Effective Resume Writing
- HR Interview Questions
- Computer Glossary
- Who is Who
What is the Plum Pudding Model of the Atom?
There were several theories stated to explain the arrangement of subatomic particles. The Plum Pudding Model, also known as Thomson's Plum Pudding Model, is also a scientific model for explaining the arrangement of subatomic particles. This model was first proposed by a British physicist Sir J. J. Thomson in 1904. This model was stated soon after the discovery of the electron, but prior to the discovery of the nucleus of the atom.
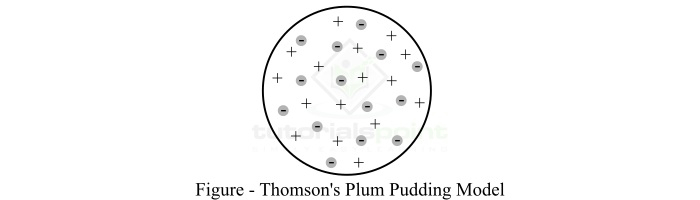
According to the plum pudding model, "a volume of positive charge surrounds electrons in an atom, like negatively charged plums embedded in a positively charged pudding." Hence, it is named the plum pudding model.
The Plum Pudding Model tried explain the following two properties of atoms ?
Electrons are negatively charged particles.
Atoms have no resultant or net electric charge because the negative charge and positive charge are equal in magnitude.
For many years, it had been known that an atom contains negatively charged subatomic particles. At the time of discovery, Sir J. J. Thomson called these negatively charged particles corpuscles.
First Model to Represent the Atomic Structure of the Matter
The Thomson's Plum Pudding Model was the first ever model to represent the atomic structure of matter. In this model, it was assumed that a matter or substance consists of small spheres that have a size of the order 10?10 meters in diameter.
In the Thomson's atomic model, the positive charge is considered to be distributed uniformly throughout the volume of the sphere and this sphere is called pudding, and the negatively charged particles, i.e., electrons are called plums are considered to be distributed as point charges in shells.
The plum pudding model is compared to a watermelon, where the seeds of the watermelon are considered as negatively charged particles and the red part of the watermelon considered as positively charged sphere.
According to this model, the positively charged sphere exerts a force on the negatively charged particles or electrons. The resultant force on the negatively charged particles is directed towards the center of the positively charged sphere. Also, the negatively charged electrons repel each other and form the shells.
The Thomson's plum pudding model was followed for few years until the discovery of the Ernest Rutherford's nuclear model of the atom in 1911. After the announcement of Ernest Rutherford's model, the validity of the Thomson's plum pudding model decreased rapidly. However, the Thomson's plum pudding model is considered as the first modern attempt to explain the theory of atomic structure.
Limitations of Thomson's Plum Pudding Model
The following are the major reasons of the failure of the Thomson's plum pudding model ?
The Thomson's plum pudding model failed to explain the stability of an atom.
This model failed to explain how the positive charge holds the negatively charged particles in the atom.
This model is not able to give the position of the nucleus in the atom.
The plum pudding model does not have any experimental evidence in its support.
The plum pudding model also failed to explain the scattering of alpha particles by thin metal foils.
This model also failed to explain the existence of the light spectrum and the emission of electron spectrum.
Due to these reasons, the Thomson's plum pudding got rejected, although this model had paved the way for the study of the atom and its structure.

