
 Data Structure
Data Structure Networking
Networking RDBMS
RDBMS Operating System
Operating System Java
Java MS Excel
MS Excel iOS
iOS HTML
HTML CSS
CSS Android
Android Python
Python C Programming
C Programming C++
C++ C#
C# MongoDB
MongoDB MySQL
MySQL Javascript
Javascript PHP
PHP
- Selected Reading
- UPSC IAS Exams Notes
- Developer's Best Practices
- Questions and Answers
- Effective Resume Writing
- HR Interview Questions
- Computer Glossary
- Who is Who
Working Principle of Voltaic Cell (Galvanic Cell)
A Voltaic cell is an electrochemical cell that converts the chemical energy of spontaneous (natural) redox reactions into electrical energy. The Voltaic cell is also called as Galvanic cell.
The voltaic cell is named after its inventor Alessandro Volta in 1799.
In redox (oxidation-reduction) reactions, the electrons are moved between two different species and if these reactions occur spontaneously then energy is released as a result of these reactions. Therefore, the released energy is used to do work. To deal with this energy, it is necessary to split the reaction into two half reactions – Oxidation and Reduction. By using two different containers and connecting wires the electrons are transferred from one end to the other end. This creates a Galvanic Cell or Voltaic Cell.
Principle of Voltaic Cell
The working principle of Voltaic cell is based on the fact that, the electric work is done by a voltaic cell due to the Gibbs free energy of spontaneous redox reactions in the voltaic cell.
The voltaic cell consists of two half cells and a salt bridge. Each half cell has a metallic electrode dipped into an electrolyte. These two half cells are connected to a voltmeter with the help of connecting wires.
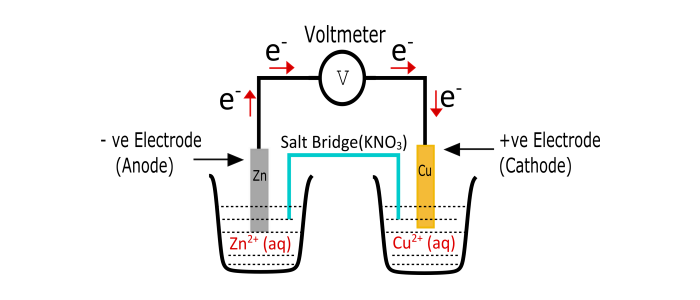
Parts of Voltaic Cell
Cathode – The cathode of voltaic cell is made up of copper. This is the positive terminal of the cell and Reduction occurs at this electrode.
Anode – This electrode is made of Zink metal. It forms negative electrode of the cell and Oxidation occurs at this electrode.
Two Half Cells – Oxidation and Reduction are separated into two different sections.
Salt Bridge – it has electrolytes required to complete the circuit in the voltaic cell.
External Circuit – through this flow of electrons takes place between the electrodes.
Working of Voltaic Cell
In a voltaic cell, when an electrode is dipped into the electrolyte at the contact surface of electrode and electrolyte, the atoms of the electrode have a tendency to generate positive ions in the electrolytic solution and leaving the electrons at the electrode. Hence, the electrode becomes negatively charged.
At the same time, the positive ions of electrolytic solution also have a tendency to deposit on the electrode and makes the electrode positively charged.
Therefore, the two opposite reactions makes the electrodes positively or negatively charged with respect to the electrolyte. Thus, a potential difference is established between the electrode and electrolyte. This potential difference is called as Electrode Potential.
Now, the electrode at which reduction takes places is called as cathode and becomes positive with respect to the solution, while the electrode at which oxidation takes place is called anode and has a negative potential with respect to the electrolytic solution. As a result of this, a potential difference established between the two electrodes of the voltaic cell. This potential difference is called as Cell Potential.
When there is no current taken from the voltaic cell, the cell potential is referred as Electromotive Force (EMF) of the voltaic cell. When an external circuit is connected to the voltaic cell, the electrons starts to flow from the anode (negative electrode) to the cathode (positive electrode). Therefore, the conventional current flows from positive terminal to the negative terminal in the external circuit.
Types of Voltaic Cell
-
Primary Cell
Dry Cell
Mercury Cell
Alkaline Cell
-
Secondary Cell
Nickel-Cadmium Cell
Lead-Acid Cell
Lithium-Ion Cell
Primary Voltaic Cell
A cell which produces electrical energy without being previously charged by some external source of electricity is called Primary Cell. In this type of cell electrical energy is obtained by the chemical reactions as long as the active material is present. Once the primary cell is used up it cannot be recharged again.
Secondary Voltaic Cell
Secondary voltaic cell once used can be recharged again by passing an electric current in the reverse direction. Therefore, it can be used again and again. The chemical changes occur when the cell is charged and these changes are reversed during discharging.
Advantages of Voltaic Cell
Primary voltaic cells produces a steady current and are lightweight, therefore, they are portable.
Secondary cells are rechargeable.
Secondary cells provide a large and steady amount of electrical energy for a long time.
Disadvantages of Voltaic Cell
Primary voltaic cells cannot be recharged.
Secondary cells are expensive.
Applications of Voltaic Cell
Used in watches, remote controllers, calculators, toys etc.
Also used in cameras, cell phones, laptops etc.
Used as a fuel cells for engines to power up.

