
 Data Structure
Data Structure Networking
Networking RDBMS
RDBMS Operating System
Operating System Java
Java MS Excel
MS Excel iOS
iOS HTML
HTML CSS
CSS Android
Android Python
Python C Programming
C Programming C++
C++ C#
C# MongoDB
MongoDB MySQL
MySQL Javascript
Javascript PHP
PHP
- Selected Reading
- UPSC IAS Exams Notes
- Developer's Best Practices
- Questions and Answers
- Effective Resume Writing
- HR Interview Questions
- Computer Glossary
- Who is Who
Sources of Electromotive Force (EMF)
Concept of Electromotive Force (EMF)
The electromotive force (EMF) of a source, is a measure of the energy the source gives to each coulomb of charge. The EMF is measured in volts (V).
At first sight, the name EMF implies that it is a force that causes the current to flow but this not correct, because it is not a force but energy supplied to the charge by some source of energy such as a battery. The EMF maintains potential difference while the potential difference causes current to flow.
Difference between EMF and Potential Difference
As we know, the EMF of the battery is the total amount of energy that the battery gives to each coulomb of charge. Thus, in the figure, if the battery supplies 4 joules of energy per coulomb of charge, then it has an EMF of 4 volts. This is the amount of energy given to each coulomb in a battery due to chemical action.
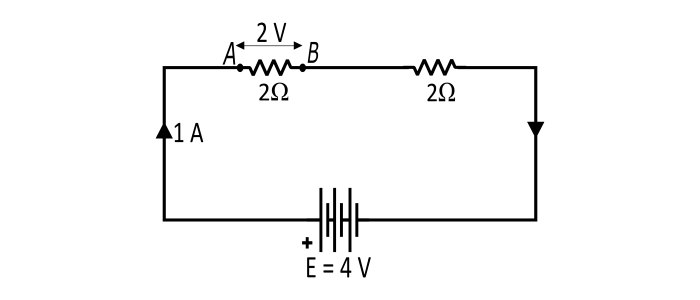
The potential difference between two points (say A and B), is a measure of energy used by one coulomb in moving from A to B. Hence, if the potential difference between points A and B is 2 volts, it means that each coulomb will give up an energy of 2 joules in moving from A to B. Hence, the potential difference between any two points is the energy used by one coulomb in moving from one point to another.
As, the each coulomb travels from positive terminal to the negative terminal of the battery through the resistances, it gives up its most of energy to the resistances and remaining to the connecting wires. When it reaches to negative terminal, it has lost all its energy supplied by the battery. Now, the battery supplies fresh energy to each coulomb to start the journey once again.
Therefore, The EMF maintains the potential difference while the potential difference causes current to flow.
Sources of Electromotive Force (EMF)
A source of EMF can be considered as a kind of charge pump that works to move charges from the point of high charge density to a point of low charge density. The source of EMF performs work (dW) on the charge to move it to the region of low charge density. Hence the EMF of the source can also be defined as the work done per unit charge i.e.
$$E=\frac{dW}{dq}$$
Chemical Sources of EMF
The batteries convert chemical energy into electrical energy. Generally, a battery is made up of two electrodes (positive and negative) and an electrolytic solution. Atoms in molecules are held together by the means of chemical bonds. When molecules of comparatively high energy are brought together, a spontaneous chemical reaction occurs that rearranges the bonding and reduces the energy of the system. In batteries, the redox reactions take place, in which a gain of electrons (i.e. reduction) occurs at one conductive electrode and loss of electrons (i.e. oxidation) at another electrode. The overall spontaneous reaction can occur only when the electrons moves between the electrodes through an external circuit. The electrical energy given off is the free energy lost by the chemical reaction.
Electromagnetic Induction
The electromagnetic induction is production of electric field by a varying magnetic field. When the magnetic flux linking a conductor or coil changes, an EMF is induced in it. Depending on the way in which the changes in the magnetic flux linkage are brought about, the EMF is of two types −
The conductor is stationary and the magnetic field is changing (as in transformer). The EMF induced in this way is called as Statically Induced EMF. It is so called because the EMF induced in a conductor which is stationary.
When the conductor is moving in a stationary magnetic field (as in generator) in such a way that the magnetic flux linking it, changes. The induced EMF in this way is called as Dynamically Induced EMF.
Solar Cell
A solar or Photovoltaic (PV) cell converts the sunlight into the electrical energy. The solar cells are made up of light sensitive semiconductor materials. The operation of solar cell is based on the photovoltaic effect i.e. the light of sufficient energy falling on the surface of semiconductor material, creates mobile electron-hole pairs in the semiconductor.
The charge separation takes place due to a pre-existing electric field, created from a built-in potential that arises from the contact potential between the two different materials in the pn-junction. The charge separation between the negative electrons and positive holes cross the pn-junction produces a DC voltage (known as Photo Voltage) between the terminals of the solar cell. This photo voltage can drive a current through the load attached across the terminals. The Photo Voltage is also referred as the Photo EMF.
Contact Potential
When two dissimilar metals are in contact, thermodynamic equilibrium needs that one of the metals considered at a higher electric potential than the other. This is called as the Contact Potential or Contact Electromotive Force. The EMF generated by thermocouples is the contact EMF i.e. when two dissimilar metals are joined and there is difference in the temperature of the metals then a DC voltage will be generated between the hot junction and the cold junction.
Piezoelectric Effect
A piezoelectric substance is one which produces an EMF when a mechanical pressure is applied to it. The quartz crystal is an example of piezoelectric. The piezo gas igniter is a common application of the piezoelectric effect.

