Data Structure
Data Structure Networking
Networking RDBMS
RDBMS Operating System
Operating System Java
Java MS Excel
MS Excel iOS
iOS HTML
HTML CSS
CSS Android
Android Python
Python C Programming
C Programming C++
C++ C#
C# MongoDB
MongoDB MySQL
MySQL Javascript
Javascript PHP
PHP
- Selected Reading
- UPSC IAS Exams Notes
- Developer's Best Practices
- Questions and Answers
- Effective Resume Writing
- HR Interview Questions
- Computer Glossary
- Who is Who
Electron Theory of Matter and Atoms
The electron theory of matter is one of the most successful and experimentally proved theory that can explain the nature of electricity. Although the study of electricity has been attracting the attention of scientists for several hundred years, and there were several experiments and theories developed to understand the nature of electricity. The only theory that has explained it successfully is the electron theory of matter.
The electron theory of matter is the result of experiments and researches conducted by many scientists like J. J. Thomson, R. A. Millikan, Earnest Rutherford and Bohr. This article is meant for explaining the concept of the electron theory of matter and the atom.
Electron Theory of Matter
The nature of electricity can be easily explained by the electron theory of matter. This theory states that all substances whether solid, liquid or gas is composed of small particles called molecules. Where, a molecule is in turn made up of minute particles called atoms.
According to the electron theory of matter,
The substance whose molecules consist of same type of atoms is called element. For example, oxygen is an element because its molecule has two atoms of same type.
The substance whose molecules consist of different kind of atoms is called compound. For example, water is a compound because its molecules contains two atoms of hydrogen and one atom of oxygen.
The Atom
The atom is the basic building block of the substance. Basically, atoms are the sub-parts of molecules. An atom consists of two parts namely,
- Nucleus
- Extra-nucleus
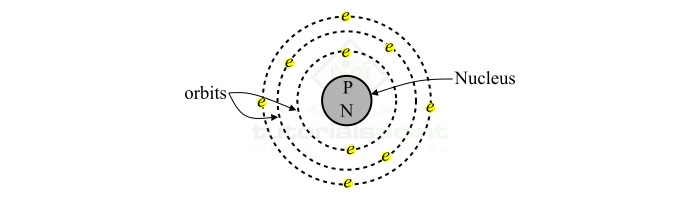
Nucleus
The nucleus is the central part of an atom and it contains two subatomic particles namely protons and neutrons. The proton is a positively charged particle. The magnitude of charge on a proton is equal to $\mathrm{+1.67\times 10^{-27}C}$ . A proton is having a mass of $\mathrm{+1.67\times 10^{-27}} kg. Another particle inside the nucleus is neutron. A neutron is an electrically neutral particle which means it does not carry any charge. Though, the mass of a neutron is equal to that of the proton. Therefore, the nucleus of an atom bears a positive charge.
Extra-Nucleus
The extra-nucleus is the outer part of an atom, i.e. it is the space in an atom around the nucleus. The extra-nucleus contains electrons only. Thus, an electron is also a subatomic particle. An electron carries a negative charge of magnitude equal to $\mathrm{-1.67\times 10^{-19}C}$ . The mass of an electron is equal to $\mathrm{9.1\times 10^{-31}}$ kg. Here, we can observe that the mass of an electron is very small as compared to that of a proton or a neutron. Therefore, the nucleus of an atom constitute the entire weight of atom.
Electrons in an atom move around the nucleus in different paths (orbits). These electrons obey the following rules while moving in their orbit ?
The maximum number of electrons that an orbit can have is equal to $\mathrm{2n^{2}}$, where n is the number of the orbit. Thus, the first orbit has 2 electrons, the second orbit has 8 electrons and so on.
The outermost (last) orbit can have maximum 8 electrons.
No orbit cannot accommodate more than 18 electrons.
In the normal state of an atom, the number of electrons is equal to the number of protons. Therefore, under normal conditions, an atom is electrically neutral as a whole and does not exhibit electricity.
The following are two important measure of an atom ?
Atomic Number ? The number of electrons or protons in an atom is known as atomic number, i.e.,
Atomic number = No. of electrons or No. of protons
Atomic Weight ? The sum of the number of protons and number of neutrons in the nucleus of an atom is known as atomic weight, i.e.,
Atomic weight = No. of protons + No. of neutrons
Conclusion
In this article, we discussed the electron theory of matter and the concept of the atom. These two concepts play a vital role in understanding the nature of electricity. The electron theory of matter is the only theory that has survived over the years to explain the nature of electricity. It also helps in understanding the basic structure of the atom.