Difference Between Central Tolerance and Peripheral Tolerance
Introduction
Normally our immune system shows a response towards the foreign antigen and does not act against self-antigens. This phenomenon is known as self-tolerance. It refers to the lack of responsiveness to the individual's self-antigens. Hence tolerance is antigen-specific.
Immune Response
Immune tolerance or immunological tolerance is the state of unresponsiveness of the immune system to the substances or tissues that are capable to induce an immune response. It differs from the conventional methods of immune-mediated elimination of foreign antigens.
Due to random genetic recombinations, immune cells genetically express receptors for the specific self and foreign antigens in the central lymphoid organs.
Tolerance can be classified into 2 types depending on where this state is originally induced in the thymus or bone marrow or in other tissues and lymph nodes.
Primary lymphoid organs (thymus and bone marrow) - Central tolerance
Peripheral sites (spleen and lymph nodes) - Peripheral tolerance
The mechanisms by which these forms of tolerance are established are distinct but the resultant effect is similar.
Central Tolerance
It is the developmental stage within the large cycle of lymphocyte maturation and it is going to make sure that these lymphocytes can distinguish self and non-self-cells which is very important to prevent auto-immune diseases. It develops during the selection of immature lymphocytes where they encounter self-antigens within the primary lymphatic organs (thymus and bone marrow).
Here the mechanisms involve the removal of self-recognizing cells that can attack which includes
Self-reactive cytotoxic T cells
Self-reactive antibody-secreting plasma cells
Central tolerance processes of negative selection and receptor editing work to eliminate many auto-reactive T cells in the thymus and auto-reactive B cells in bone marrow respectively.
Negative Selection
Results in the induction of death or apoptosis in some cells that carry potentially active TCRs or BCRs.
This selection removes the T or B cell clones before they attain maturation if they possess receptors that recognize self-antigens and bind to them with strong affinity.
This mainly occurs in the primary lymphoid organs, bone marrow for B cells and thymus for T cells.
Receptor Editing
V region which is specific to antigens in developing B cells usually undergoes receptor editing.
In this process, the V region is edited or switched for a different V region gene segment through VDJ genetic recombination.
This mechanism produces the less reactive auto receptors with a moderate affinity for self-antigens that would lead to disease allowing the cells to survive.
Peripheral Tolerance
This occurs when the mature lymphocytes become incapable of responding to the antigens. Peripheral tolerance is the key to preventing the over-reactivity of the immune system to various environmental entities.
Here the mechanism involves selecting self-recognizing cells that can protect which relies on a subset of T cells, Treg cells that inhibit immune responses against self-antigens and foreign antigens.
Two factors explain why we need this tolerance ?
Not all self-antigens are expressed in the central lymphoid organs where the negative selection occurs.
Affinity to self-antigens requires a threshold level before clonal selection is triggered allowing the weakly self-reactive clones to survive this process.
Peripheral tolerance is mediated by 3 different mechanisms
Anergy
Treg-mediated suppression
Apoptosis
Anergy
T and B cells need induction of costimulatory activation apart from the MHC-induced activation. T cells co-stimulation requires receptor CD28/B7 and B cell co-stimulation requires receptor CD40. Inhibition of such co-stimulation leads the T cells to become anergic which is an unresponsiveness state.
Treg-Mediated Suppression
Some of the mechanisms for peripheral tolerance involve the T regulatory cells (subsets of CD4 and CD8). In the thymus, the thymocytes which have a high affinity for self-antigens undergo apoptosis leading to their death, whereas the thymocytes with a low and moderate affinity towards the self-antigens cross the barrier and enter the periphery during negative selection hence we need peripheral tolerance as well.
T cells with moderate affinity for self-antigen generate T regulatory cells or Treg cells during negative selection with an upregulation of transcription factor FOXP3 and enter the periphery where they inhibit or down-regulate the proliferation of self-reactive T cells.
Most of the Tregs are a unique subset of CD4 T cells that expresses high levels of IL-2R chain (CD25), which secretes IL10 and TGF beta. These can inhibit self-reactive lymphocytes.

Stimulation of the Regulatory T cells illustration
Importance of Treg Cells
TREG cells recognize specific self-antigens and foreign antigens through the TCR interactions.
Down-regulates the immune processes when they react with self-antigens in the peripheral sites.
It can suppress the immune responses towards the non-self-antigens.
Tregs have high levels of CTLA4 or cytotoxic T lymphocyte antigen-4 protein. These CTLA4 have high affinity for B7 which is present on antigen-presenting cells than CD28 and blocks the co-stimulatory signal of B7.
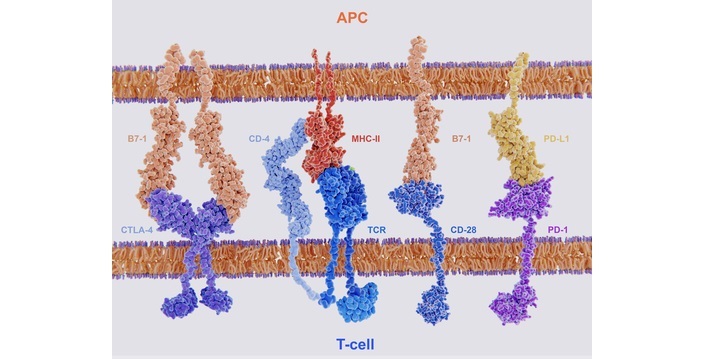
Membrane proteins involved in the activation and inhibition
Lack of Treg cells may develop a syndrome called IPEX (immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome).
Apoptosis
Where the T cells undergo activation-induced cell death. Done by 2 pathways
Mitochondrial or Intrinsic Pathway ? regulated by the Bcl-2 family of proteins, this occurs by the activation of caspase cascade leading to DNA fragmentation when the activating Bim (pro-apoptotic molecule) receptor binds to the Bax and Bak and this insert into mitochondrial membrane resulting in the leaking of cytochrome c into the cytoplasm.
Death Receptor or Extrinsic Pathway ? Cell surface receptors Fas and FasL, are engaged by ligands leading to the activation of the caspase cascade.
In B cell peripheral tolerance, self-reactive B cells require high levels of growth factor BAFF/BLys for survival. High-affinity cells may undergo apoptosis and low-affinity cells may signal through inhibitory receptors.
Tolerance however has its negative tradeoffs. It allows for some pathogenic microbes to successfully infect a host and avoid elimination. In addition, inducing peripheral tolerance in the local microenvironment is a common survival strategy for the number of tumors that prevents their elimination by the host immune system.
Deficits in central and peripheral tolerance also cause auto-immune disease resulting in syndromes like systemic lupus erythematosus, rheumatoid arthritis, type I diabetes, auto-immune polyendocrine syndrome type I and immunodysregulation polyendocrinopathy, enteropathy, X-linked syndrome and potentially contribute to asthma, allergy, and inflammatory bowel disease.
Conclusion
Normally autoimmune diseases are prevented in the body when our immune system recognizes the self-antigen from the foreign antigen and is non responsive towards the self-antigen. This is called as immunological tolerance which is the normal state of our body.
This immune tolerance is of 2types central tolerance which occurs in the primary lymphoid organs (thymus and bone marrow) where the negative selection result in the removal of reactive-T cells which bind to the self-antigens and peripheral tolerance which occurs in the peripheral sites like spleen and lymph node where it involved TREG cells which inhibits the immune responses towards the self-antigens.


 Data Structure
Data Structure Networking
Networking RDBMS
RDBMS Operating System
Operating System Java
Java iOS
iOS HTML
HTML CSS
CSS Android
Android Python
Python C Programming
C Programming C++
C++ C#
C# MongoDB
MongoDB MySQL
MySQL Javascript
Javascript PHP
PHP