Article Categories
- All Categories
-
 Data Structure
Data Structure
-
 Networking
Networking
-
 RDBMS
RDBMS
-
 Operating System
Operating System
-
 Java
Java
-
 MS Excel
MS Excel
-
 iOS
iOS
-
 HTML
HTML
-
 CSS
CSS
-
 Android
Android
-
 Python
Python
-
 C Programming
C Programming
-
 C++
C++
-
 C#
C#
-
 MongoDB
MongoDB
-
 MySQL
MySQL
-
 Javascript
Javascript
-
 PHP
PHP
Difference between Galvanic Cell and Electrolytic Cell
A cell is a device which can convert chemical energy into electrical energy. A cell consists of two electrodes and chemical substances inside it. When the cell is connected in a circuit, the chemical reactions take place inside the cell and as a result of these chemical reactions, an electric current flows in the circuit.
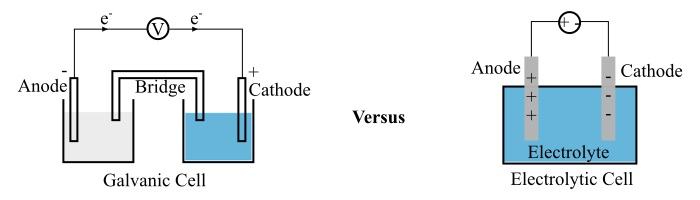
In this article, we will discuss about galvanic cells and electrolytic cells and how these two types of cells are different from each other.
What is a Galvanic Cell?
A galvanic cell is an electrochemical cell in which an electric is produced from spontaneous oxidation-reduction reactions.
The galvanic cell consists of two half cells, where each half cell contains an electrode in an electrolyte. The two electrodes are separated from each other to prevent a direct chemical contact of the oxidation and reduction reactions by creating a potential difference.
When the circuit of the galvanic cell is closed, the electrons that are released in the oxidation reaction travels through the external circuit to reach another electrode and finally these electrons are used by the reduction reaction. In this way, galvanic cell function to establish an electric current in the external circuit.
What is an Electrolytic Cell?
An electrolytic cell is also an electrochemical cell that uses electrical energy from some external source to drive a chemical reaction.
A typical electrolytic cell has three major parts namely two electrodes (anode and cathode) and an electrolyte. Where, the electrolyte is a solution of water and molten slat such as sodium chloride in which ions are dissolved
When a voltage is applied to the electrodes, the ions of the electrolyte are attracted towards an electrode with opposite polarity. Electrolytic cells are extensively used in electrolysis to decompose the chemical compounds.
Difference between Galvanic Cell and Electrolytic Cell
Both galvanic cells and electrolytic cells are types of electrochemical cells. However, there are many differences between a galvanic cell and an electrolytic cell that are listed in the following table ?
| Basis of Difference | Galvanic Cell | Electrolytic Cell |
|---|---|---|
| Definition | A galvanic cell is an electrochemical cell in which chemical energy is transformed into electrical energy. | An electrolytic cell is an electrochemical cell in which electrical energy is transformed into chemical energy. |
| Energy conversion | In a galvanic cell, chemical energy is converted into electrical energy. | In an electrolytic cell, electrical energy is converted into chemical energy. |
| Electrode polarity | For a galvanic cell, the positive terminal is called cathode, while the negative terminal is called anode. | In case of an electrolytic cell, the anode is positive and cathode is negative. |
| Flow of electrons | Electrons being negatively charged flow from anode to cathode of the galvanic cell. | Electrons flow from cathode to anode in an electrolytic cell. |
| Chemical reaction | A spontaneous chemical reaction occurs in a galvanic cell to release electrical energy. | In an electrolytic cell, non-spontaneous chemical reactions take place. |
| Need of external voltage source | A galvanic cell does not require external voltage source. | An external voltage source is required in an electrolytic cell. |
| Electrical energy flow | Electrical energy flows from cell to the external circuit in a galvanic cell. | In case of electrolytic cell, electrical energy flows from external circuit to the cell. |
| Number of cell containers | Galvanic cell requires two separate cell containers that form two half cells connected by a salt bridge. | A single cell container forms the complete electrolytic cell. |
| Ions on electrodes | In a galvanic cell, ions discharge at the cathode and consume at the anode. | Both the electrodes of an electrolytic cell discharge the ions. |
| Oxidation & reduction reactions | In a galvanic cell, the oxidation reaction takes place at anode while the reduction reaction takes place at the cathode. | In an electrolytic cell, the oxidation reaction occurs at the cathode and the reduction reaction occurs at the anode. |
| Applications | Galvanic cells are used as the source of electricity, used in batteries, etc. | Electrolytic cells are used in electrolysis for purifying metals, etc. |
Conclusion
The most significant difference between a galvanic cell and an electrolytic cell is that a galvanic cell converts chemical energy into electrical energy, whereas an electrolytic cell converts electrical energy into chemical energy.


