
 Data Structure
Data Structure Networking
Networking RDBMS
RDBMS Operating System
Operating System Java
Java MS Excel
MS Excel iOS
iOS HTML
HTML CSS
CSS Android
Android Python
Python C Programming
C Programming C++
C++ C#
C# MongoDB
MongoDB MySQL
MySQL Javascript
Javascript PHP
PHP
- Selected Reading
- UPSC IAS Exams Notes
- Developer's Best Practices
- Questions and Answers
- Effective Resume Writing
- HR Interview Questions
- Computer Glossary
- Who is Who
Difference between Electron and Proton
According to the Electron Theory of Matter, ever matter is composed of minute particles called molecules. A molecule is in turn made up of atoms. Therefore, the atom is the basic building block of the matter.
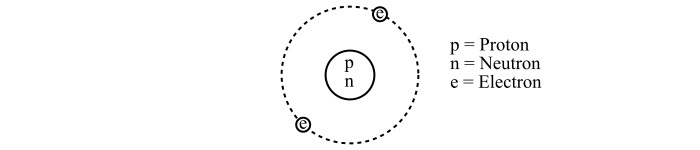
An atom consists of two main parts viz. nucleus and extra-nucleus. The nucleus is the central part of the atom and has two subatomic particles viz. proton and neutron. Whereas, the extra-nucleus is the space around the nucleus in which the electrons revolve around the nucleus in different orbits.
In this article, we are going to enlist all the major differences between electron and proton. Although, both electron and proton are sub-atomic particles, these are also called particles of electricity because they act as the charge carrier in substances.
What is Electron?
Electron is a sub-atomic particle which carries an electric charge of negative polarity. Inside the atom, the electrons are found in the extra-nucleus of the atom and moves around the nucleus in the orbit.
Electrons are represented by the symbol ‘e’. The negative electric charge carried by an electron is equal to -1.6 × 10-19 C. The mass of an electron is 9.1 × 10-31 kg. A British Physicist J. J. Thompson discovered electron in 1897. Electron is the particle which led to the explanation of several phenomena in science such as electricity, magnetism, chemical bonding, thermal conductivity, etc.
What is Proton?
Proton is a sub-atomic particle which carries an electric charge of positive polarity. The proton lies in the nucleus of the atom which makes the nucleus positively charged.
The proton is usually denoted by the symbol ‘p’. The credit of discovery of proton goes to Ernest Rutherford, who presented that every atom has a positively charged particle in its nucleus in the year 1917. The charge carried by a proton is equal to that of electrons, i.e., +1.6 × 10-19 C and the mass of proton is equal to 1.67 × 10-27 kg.
Difference between Electron and Proton
Both proton and electron are the subatomic particles of an atom which carries electric charge of positive and negative polarity respectively. However, there are many differences between proton and electron which are highlighted in the following table
Basis of Difference |
Electron |
Proton |
|---|---|---|
Definition |
An electron is a sub-atomic particle that holds negative electric charge. | A proton is a sub-atomic particle that holds positive electric charge. |
Representation |
Electron is generally denoted by the symbol ‘e’. | Proton is usually denoted by the symbol ‘p’. |
Polarity of electric charge |
Electron carries the electric charge of negative polarity. | Proton carries the electric charge of positive polarity. |
Value of electric charge |
Charge on one electron is equal to -1.6 × 10-19 C. | Charge on one proton is equal to +1.6 × 10-19 C. |
Mass |
The mass of electron is less than that of a proton and is equal to 9.1 × 10-31 kg. | The mass of a proton is more than that of an electron and is equal to 1.67 × 10-27 kg. |
Location inside the atom |
Electron is present in the space around the nucleus, called extra-nucleus. | Proton is present inside the nucleus of the atom. |
Mobility |
Electrons can freely move in a well-defined orbit around the nucleus of the atom. | Proton does not exhibit mobility because they present inside the nucleus and strongly hold by the nuclear forces. |
Addition or removal |
Electrons can be easily added to or removed from an atom. | It is very hard to add or remove the proton in an atom. |
Involvement in reactions |
Electrons are involved in the chemical reactions. | Protons are not usually involved in the normal chemical reactions, but they can be involved in the nuclear reactions. |
Involvement in the flow of electric current |
Electrons are entirely responsible for the flow of electric current. | Protons do not involve in the flow of electric current. |
Conclusion
Both electron and proton are the sub-atomic particles in an atom. All the major differences between electron and proton are listed in the above table. The most significant difference is that electron is a negatively charged particle which present in the orbits around the nucleus, whereas a proton is a positively charged particle present inside the nucleus of the atom.

