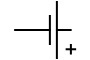Data Structure
Data Structure Networking
Networking RDBMS
RDBMS Operating System
Operating System Java
Java MS Excel
MS Excel iOS
iOS HTML
HTML CSS
CSS Android
Android Python
Python C Programming
C Programming C++
C++ C#
C# MongoDB
MongoDB MySQL
MySQL Javascript
Javascript PHP
PHP
- Selected Reading
- UPSC IAS Exams Notes
- Developer's Best Practices
- Questions and Answers
- Effective Resume Writing
- HR Interview Questions
- Computer Glossary
- Who is Who
Difference between Cell and Battery
Both cell and battery are the sources of electrical energy in any electrical circuit that provide necessary excitation to the circuit. The cell and battery always deliver electric power to the circuit in the form of direct current (DC), they can never deliver alternating current (AC). Therefore, in practice, the cell and battery are the primary sources of direct current (DC) electricity. However, the working principle of both cell and battery is the same, i.e. electrochemical reactions where the electrical energy is converted into chemical energy and vice-versa.
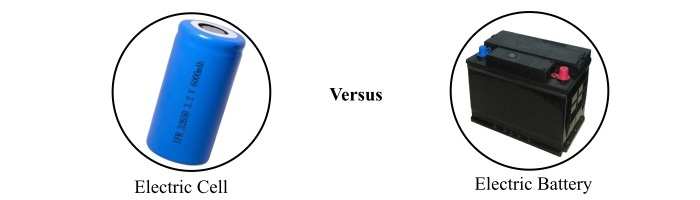
In this article, we will compare cell and battery by considering various parameters such as basic definition, types, uses, size, cost, etc. We will also provide a basic introduction of cell and battery so that the understanding of the differences between them becomes easier.
What is a Cell?
A cell or electric cell is a device that can supply direct current electricity to an electric circuit. The cell converts the chemical energy stored in it into electrical energy. The cells has two terminals or electrodes viz. anode and cathode. These cell electrodes react with an electrolyte (a chemical substance) to produce electrical energy.
Depending on the ability to recharge, the cells are of two types as ?
Primary cell
Secondary cell
The primary cell is the one that can produce electricity once and then discarded. Whereas, a secondary cell is the one that can be recharged many times to produce electrical energy. Hence, a cell that has ability to recharge is called secondary cell.
The cells are generally used in portable devices such as clocks, remote controls, toys, torches, etc.
What is a Battery?
A battery is a type of energy source which consists of two or more cells connected together and sealed in a single unit. Like a cell, the battery also has two terminals viz. anode and cathode.
The battery is also an electrochemical device, i.e. it involves chemical reactions to produce electrical energy. The battery is mainly used in a device that requires more amount of electrical energy to function.
Like a cell, the battery is also of two types based their charging ability as ?
Primary battery
Secondary battery
The primary battery is the one that involves irreversible chemical reaction and cannot be recharged after used once. Whereas, in a secondary battery, the chemical reaction takes place is reversible that means it can be recharged many times.
Difference between Cell and Battery
Since both cell and battery are the two major sources of direct current electricity. However, there are many differences between a cell and a battery that are listed in the following table ?
| Basis of Difference | Cell | Battery |
|---|---|---|
| Definition | A cell is an active circuit element that converts chemical energy to produce electrical energy. | A battery is a collection of two or more cells connected together in a single unit and produces electrical energy by performing a chemical reaction. |
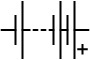
| Circuit symbol |
|
|
| Types | Cells are of two types ? primary cell and secondary cell. | Types of battery are: primary battery and secondary battery. |
| Service time period | Cell supplies electrical power for a short period of time. | Battery supplies electrical power to the circuit for a long duration. |
| Physical size | The size of a cell is small. | Battery is relatively larger in size. |
| Weight | Cell is light in weight. | Battery is heavy. |
| Cost | The cost of a cell is quite low. | Battery is relatively costlier. |
| Energy supplied | Cell can supply only small amount of energy for short time. | Battery can provide more amount of energy than a cell, because it consists of many cells in a single unit. |
| Examples | Dry cell, Daniel cell, electrolytic cell, fuel cell, galvanic cell, Leclanche cell, etc. | Li-Ion battery, lead-acid battery, Ni-Cd battery, etc. |
| Application | Cells are generally used in portable devices like clock, torch, toys, remote controls, etc. | Batteries are used in devices that demand more power to operate such as lamps, inverters, automobiles, emergency lights, etc. |
Conclusion
The most significant difference between a cell and a battery is that a cell is a small single unit that converts chemical energy into electrical energy, whereas a battery is a group of two or more cells that also converts chemical energy into electrical energy.